Mechanism of origin of conduction disturbances in aging human atrial bundles: experimental and model study
- PMID: 17275753
- PMCID: PMC1847326
- DOI: 10.1016/j.hrthm.2006.10.023
Mechanism of origin of conduction disturbances in aging human atrial bundles: experimental and model study
Abstract
Background: Aging is associated with a significant increase in atrial tachyarrhythmias, especially atrial fibrillation. A macroscopic repolarization gradient created artificially by a stimulus at one site before a premature stimulus from a second site is widely considered to be part of the experimental protocol necessary for the initiation of such arrhythmias in the laboratory. How such gradients occur naturally in aging atrial tissue is unknown.
Objective: The objective of this study was to determine if the pattern of cellular connectivity in aging human atrial bundles produces a mechanism for variable early premature responses.
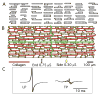
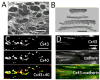
Methods: Extracellular and intracellular potentials were recorded after control and premature stimuli at a single site in aging human atrial bundles. We also measured cellular geometry, the distribution of connexins, and the distribution of collagenous septa. A model of the atrial bundles was constructed based on the morphological results. Action potential propagation and the sodium current were analyzed after premature stimuli in the model.
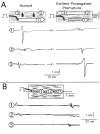
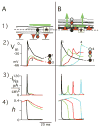
Results: Similar extracellular potential waveform responses occurred after early premature stimuli in the aging bundles and in the model. Variable premature conduction patterns were accounted for by the single model of aging atrial structure. A major feature of the model results was that the conduction events and the magnitude of the sodium current at multiple sites were very sensitive to small changes in the location and the timing of premature stimuli.
Conclusion: In aging human atrial bundles stimulated from only a single site, premature stimuli induce variable arrhythmogenic conduction responses. The generation of these responses is greatly enhanced by remodeling of cellular connectivity during aging. The results provide insight into sodium current structural interactions as a general mechanism of arrhythmogenic atrial responses to premature stimuli.
Figures




Comment in
-
Aging and atrial fibrillation research: where we are and where we should go.Heart Rhythm. 2007 Feb;4(2):186-7. doi: 10.1016/j.hrthm.2006.11.011. Epub 2006 Nov 17. Heart Rhythm. 2007. PMID: 17275754 Free PMC article. No abstract available.
-
Re: mechanism of origin of conduction disturbances in aging human atrial bundles: experimental and model study.Heart Rhythm. 2007 Jul;4(7):989; author reply 989-90. doi: 10.1016/j.hrthm.2007.03.013. Epub 2007 Mar 23. Heart Rhythm. 2007. PMID: 17599692 Free PMC article. No abstract available.
References
-
- Spach MS, Dolber PC, Heidlage JF. Influence of the passive anisotropic properties on directional differences in propagation following modification of the sodium conductance in human atrial muscle. A model of reentry based on anisotropic discontinuous propagation. Circ Res. 1988;62:811–832. - PubMed
-
- Chen P-S, Wolf PD, Dixon EG, Danieley ND, Frazier DW, Smith WM, Ideker R. Mechanism of ventricular vulnerability to single premature stimuli in open-chest dogs. Circ Res. 1988;62:1191–1209. - PubMed
-
- Gotoh M, Uchida T, Mandel WJ, Fishbein MC, Chen P-S, Karagueuzian HS. Cellular graded responses and ventricular vulnerability to reentry by a premature stimulus in isolated canine ventricle. Circulation. 1997;95:2141–2154. - PubMed
-
- Karagueuzian HS, Chen P-S. Cellular mechanism of reentry induced by a strong electrical stimulus: Implications for fibrillation and defibrillation. Cardiovasc Res. 2001;50:251–262. - PubMed
-
- Antzelevitch C, Fish J. Electrical heterogeneity within the ventricular wall. Basic Res Cardiol. 2001;96:517–527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

