N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology
- PMID: 17275978
- PMCID: PMC1919520
- DOI: 10.1016/j.pneurobio.2006.12.003
N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology
Abstract
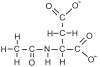
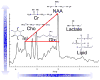
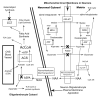
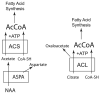
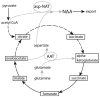
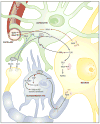
The brain is unique among organs in many respects, including its mechanisms of lipid synthesis and energy production. The nervous system-specific metabolite N-acetylaspartate (NAA), which is synthesized from aspartate and acetyl-coenzyme A in neurons, appears to be a key link in these distinct biochemical features of CNS metabolism. During early postnatal central nervous system (CNS) development, the expression of lipogenic enzymes in oligodendrocytes, including the NAA-degrading enzyme aspartoacylase (ASPA), is increased along with increased NAA production in neurons. NAA is transported from neurons to the cytoplasm of oligodendrocytes, where ASPA cleaves the acetate moiety for use in fatty acid and steroid synthesis. The fatty acids and steroids produced then go on to be used as building blocks for myelin lipid synthesis. Mutations in the gene for ASPA result in the fatal leukodystrophy Canavan disease, for which there is currently no effective treatment. Once postnatal myelination is completed, NAA may continue to be involved in myelin lipid turnover in adults, but it also appears to adopt other roles, including a bioenergetic role in neuronal mitochondria. NAA and ATP metabolism appear to be linked indirectly, whereby acetylation of aspartate may facilitate its removal from neuronal mitochondria, thus favoring conversion of glutamate to alpha ketoglutarate which can enter the tricarboxylic acid cycle for energy production. In its role as a mechanism for enhancing mitochondrial energy production from glutamate, NAA is in a key position to act as a magnetic resonance spectroscopy marker for neuronal health, viability and number. Evidence suggests that NAA is a direct precursor for the enzymatic synthesis of the neuron specific dipeptide N-acetylaspartylglutamate, the most concentrated neuropeptide in the human brain. Other proposed roles for NAA include neuronal osmoregulation and axon-glial signaling. We propose that NAA may also be involved in brain nitrogen balance. Further research will be required to more fully understand the biochemical functions served by NAA in CNS development and activity, and additional functions are likely to be discovered.
Figures


References
-
- Abbott C, Bustillo J. What have we learned from proton magnetic resonance spectroscopy about schizophrenia? A critical update. Curr Opin Psychiatry. 2006;19:135–139. - PubMed
-
- Adachi M, Schneck L, Cara J, Volk BW. Spongy degeneration of the central nervous system (van Bogaert and Bertrand type; Canavan’s disease) A review. Hum Pathol. 1973;4:331–347. - PubMed
-
- Akimitsu T, Kurisu K, Hanaya R, Iida K, Kiura Y, Arita K, Matsubayashi H, Ishihara K, Kitada K, Serikawa T, Sasa M. Epileptic seizures induced by N-acetyl-L-aspartate in rats: in vivo and in vitro studies. Brain Res. 2000;861:143–150. - PubMed
-
- Anderson KJ, Borja MA, Cotman CW, Moffett JR, Namboodiri MA, Neale JH. N-acetylaspartylglutamate identified in the rat retinal ganglion cells and their projections in the brain. Brain Res. 1987;411:172–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

