Seminal proteins but not sperm induce morphological changes in the Drosophila melanogaster female reproductive tract during sperm storage
- PMID: 17276455
- PMCID: PMC2144743
- DOI: 10.1016/j.jinsphys.2006.12.003
Seminal proteins but not sperm induce morphological changes in the Drosophila melanogaster female reproductive tract during sperm storage
Abstract
In most insects, sperm transferred by the male to the female during mating are stored within the female reproductive tract for subsequent use in fertilization. In Drosophila melanogaster, male accessory gland proteins (Acps) within the seminal fluid are required for efficient accumulation of sperm in the female's sperm storage organs. To determine the events within the female reproductive tract that occur during sperm storage, and the role that Acps and sperm play in these events, we identified morphological changes that take place during sperm storage in females mated to wild-type, Acp-deficient or sperm-deficient males. A reproducible set of morphological changes occurs in a wild-type mating. These were categorized into 10 stereotypic stages. Sperm are not needed for progression through these stages in females, but receipt of Acps is essential for progression beyond the first few stages of morphological change. Furthermore, females that received small quantities of Acps reached slightly later stages than females that received no Acps. Our results suggest that timely morphological changes in the female reproductive tract, possibly muscular in nature, may be needed for successful sperm storage, and that Acps from the male are needed in order for these changes to occur.
Figures
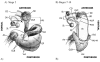




References
-
- Alonso-Pimentel H, Tolbert LP, Heed WB. Ultrastructural examination of the insemination reaction in Drosophila. Cell and Tissue Research. 1994;275:467 –479. - PubMed
-
- Arthur BI, Jr, Hauschteck-Jungen E, Nöthiger R, Ward PI. A female nervous system is necessary for normal sperm storage in Drosophila melanogaster: a masculinized nervous system is as good as none. Proceedings B of the Royal Society of London. 1998;265:1749–1753.
-
- Bairati A. Structure and ultrastructure of the male reproductive system in Drosophila melanogaster Meig. 2 The genital duct and accessory glands. Italian Journal of Zoology. 1968;2:105–182.
-
- Bairati A, Perotti ME. Occurence of a compact plug in the genital duct of Drosophila females after mating. Drosophila Information Service. 1970;45:67–68.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

