Human constitutive androstane receptor mediated methotrexate induction of human dehydroepiandrosterone sulfotransferase (hSULT2A1)
- PMID: 17276571
- PMCID: PMC1919471
- DOI: 10.1016/j.tox.2006.12.019
Human constitutive androstane receptor mediated methotrexate induction of human dehydroepiandrosterone sulfotransferase (hSULT2A1)
Abstract
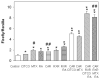
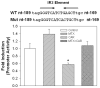
Sulfotransferases (SULTs) catalyzed sulfation is important in the regulation of biological activities of hormones and neurotransmitters, the metabolism of drugs, and the detoxification of xenobiotic toxicants. Sulfation also leads to the bioactivation of procarcinogens. Human dehydroepiandrosterone sulfotransferase (hSULT2A1) is a major SULT catalyzing the sulfation of hydroxysteroids and xenobiotic alcohols. Our previous studies had shown that the anti-folate drug methotrexate (MTX) can up-regulate several major isoforms of human SULTs. To determine the mechanisms controlling the regulation of hSULT2A1, the 5'-flanking region of hSULT2A1 was constructed into the pGL3-Basic luciferase reporter vector. The transcriptional regulation mechanism of hSULT2A1 promoter was studied using Caco-2 cell line based on the reporter gene assay. Nuclear receptor co-transfection results indicated that human constitutive androstane receptor (hCAR) and human retinoid X receptor alpha (hRXRalpha) were involved in the transcriptional regulation of hSULT2A1. RNA interference experiments further proved the role of hCAR in hSULT2A1 regulation. Progressive promoter deletion, DNA sequence alignment, and site directed promoter mutation results suggested that an imperfect inverted repeat DNA motif, IR2 (-186AGCTCAGATGACCC-173), within the hSULT2A1 promoter region mediated the hSULT2A1 induction by MTX. Furthermore, electrophoretic mobility shift assay and super shift assay were employed to characterize the interactions of hCAR and hRXRalpha with the IR2 element. In summary, we identified an IR2 DNA cis-element located at -186/-173 of hSULT2A1 promoter region; the IR2 element mediates the MTX induction of hSULT2A1 through interacting with hCAR and hRXRalpha.
Figures




References
-
- ABBAS-TERKI T, BLANCO-BOSE W, DEGLON N, PRALONG W, AEBISCHER P. Lentiviral-mediated RNA interference. Hum Gene Ther. 2002;13:2197–201. - PubMed
-
- ASSEM M, SCHUETZ EG, LEGGAS M, SUN D, YASUDA K, REID G, ZELCER N, ADACHI M, STROM S, EVANS RM, MOORE DD, BORST P, SCHUETZ JD. Interactions between hepatic Mrp4 and Sult2a as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J Biol Chem. 2004;279:22250–7. Epub 2004 Mar 5. - PubMed
-
- ASTE N, COZZI B, STANKOV B, PANZICA G. Sexual differences and effect of photoperiod on melatonin receptor in avian brain. Microsc Res Tech. 2001;55:37–47. - PubMed
-
- CHEN X, BAKER SM, CHEN G. Methotrexate induction of human sulfotransferases in Hep G2 and Caco-2 cells. J Appl Toxicol. 2005;28:28. - PubMed
-
- CHEUNG RL, LEE C, JONES EJ, RIDDICK DS. Lack of effect of methotrexate on the expression of constitutive hepatic cytochromes P450 in the male rat. Xenobiotica. 1996;26:503–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

