Intraflagellar transport protein 27 is a small G protein involved in cell-cycle control
- PMID: 17276912
- PMCID: PMC1905864
- DOI: 10.1016/j.cub.2006.12.040
Intraflagellar transport protein 27 is a small G protein involved in cell-cycle control
Abstract
Background: Intraflagellar transport (IFT) is a motility process operating between the ciliary/flagellar (interchangeable terms) membrane and the microtubular axoneme of motile and sensory cilia. Multipolypeptide IFT particles, composed of complexes A and B, carry flagellar precursors to their assembly site at the flagellar tip (anterograde) powered by kinesin, and turnover products from the tip back to the cytoplasm (retrograde) driven by cytoplasmic dynein. IFT is essential for the assembly and maintenance of almost all eukaryotic cilia and flagella, and mutations affecting either the IFT motors or the IFT particle polypeptides result in the inability to assemble normal flagella or in defects in the sensory functions of cilia.
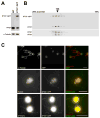
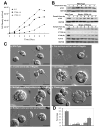
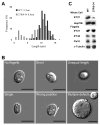
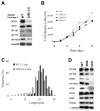
Results: We found that the IFT complex B polypeptide, IFT27, is a Rab-like small G protein. Reduction of the level of IFT27 by RNA interference reduces the levels of other complex A and B proteins, suggesting that this protein is instrumental in maintaining the stability of both IFT complexes. Furthermore, in addition to its role in flagellar assembly, IFT27 is unique among IFT polypeptides in that its partial knockdown results in defects in cytokinesis and elongation of the cell cycle and a more complete knockdown is lethal.
Conclusion: IFT27, a small G protein, is one of a growing number of flagellar proteins that are now known to have a role in cell-cycle control.
Figures

Similar articles
-
The intraflagellar transport machinery of Chlamydomonas reinhardtii.Traffic. 2003 Jul;4(7):435-42. doi: 10.1034/j.1600-0854.2003.t01-1-00103.x. Traffic. 2003. PMID: 12795688 Review.
-
Probing the role of IFT particle complex A and B in flagellar entry and exit of IFT-dynein in Chlamydomonas.Protoplasma. 2012 Jul;249(3):851-6. doi: 10.1007/s00709-011-0311-4. Epub 2011 Aug 19. Protoplasma. 2012. PMID: 21853389
-
A global analysis of IFT-A function reveals specialization for transport of membrane-associated proteins into cilia.J Cell Sci. 2019 Feb 11;132(3):jcs220749. doi: 10.1242/jcs.220749. J Cell Sci. 2019. PMID: 30659111 Free PMC article.
-
Chlamydomonas ARMC2/PF27 is an obligate cargo adapter for intraflagellar transport of radial spokes.Elife. 2022 Jan 4;11:e74993. doi: 10.7554/eLife.74993. Elife. 2022. PMID: 34982025 Free PMC article.
-
Intraflagellar transport: mechanisms of motor action, cooperation, and cargo delivery.FEBS J. 2017 Sep;284(18):2905-2931. doi: 10.1111/febs.14068. Epub 2017 Apr 18. FEBS J. 2017. PMID: 28342295 Free PMC article. Review.
Cited by
-
Intraflagellar transport: it's not just for cilia anymore.Curr Opin Cell Biol. 2010 Feb;22(1):75-80. doi: 10.1016/j.ceb.2009.10.010. Epub 2009 Dec 3. Curr Opin Cell Biol. 2010. PMID: 19962875 Free PMC article. Review.
-
A draft of the human septin interactome.PLoS One. 2010 Nov 2;5(11):e13799. doi: 10.1371/journal.pone.0013799. PLoS One. 2010. PMID: 21082023 Free PMC article.
-
Primary Cilium in Cancer Hallmarks.Int J Mol Sci. 2019 Mar 16;20(6):1336. doi: 10.3390/ijms20061336. Int J Mol Sci. 2019. PMID: 30884815 Free PMC article. Review.
-
Katanin knockdown supports a role for microtubule severing in release of basal bodies before mitosis in Chlamydomonas.Mol Biol Cell. 2009 Jan;20(1):379-88. doi: 10.1091/mbc.e07-10-1007. Epub 2008 Nov 12. Mol Biol Cell. 2009. PMID: 19005222 Free PMC article.
-
The intraflagellar transport protein IFT27 promotes BBSome exit from cilia through the GTPase ARL6/BBS3.Dev Cell. 2014 Nov 10;31(3):265-278. doi: 10.1016/j.devcel.2014.09.004. Epub 2014 Oct 30. Dev Cell. 2014. PMID: 25443296 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

