A role for Cdc2- and PP2A-mediated regulation of Emi2 in the maintenance of CSF arrest
- PMID: 17276914
- PMCID: PMC2790409
- DOI: 10.1016/j.cub.2006.12.045
A role for Cdc2- and PP2A-mediated regulation of Emi2 in the maintenance of CSF arrest
Abstract
Background: Vertebrate oocytes are arrested in metaphase II of meiosis prior to fertilization by cytostatic factor (CSF). CSF enforces a cell-cycle arrest by inhibiting the anaphase-promoting complex (APC), an E3 ubiquitin ligase that targets Cyclin B for degradation. Although Cyclin B synthesis is ongoing during CSF arrest, constant Cyclin B levels are maintained. To achieve this, oocytes allow continuous slow Cyclin B degradation, without eliminating the bulk of Cyclin B, which would induce release from CSF arrest. However, the mechanism that controls this continuous degradation is not understood.
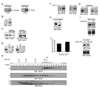
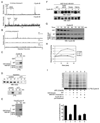
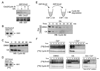
Results: We report here the molecular details of a negative feedback loop wherein Cyclin B promotes its own destruction through Cdc2/Cyclin B-mediated phosphorylation and inhibition of the APC inhibitor Emi2. Emi2 bound to the core APC, and this binding was disrupted by Cdc2/Cyclin B, without affecting Emi2 protein stability. Cdc2-mediated phosphorylation of Emi2 was antagonized by PP2A, which could bind to Emi2 and promote Emi2-APC interactions.
Conclusions: Constant Cyclin B levels are maintained during a CSF arrest through the regulation of Emi2 activity. A balance between Cdc2 and PP2A controls Emi2 phosphorylation, which in turn controls the ability of Emi2 to bind to and inhibit the APC. This balance allows proper maintenance of Cyclin B levels and Cdc2 kinase activity during CSF arrest.
Figures




References
-
- Masui Y, Markert CL. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971;177:129–145. - PubMed
-
- Sagata N, Watanabe N, Vande Woude GF, Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature. 1989;342:512–518. - PubMed
-
- Haccard O, Sarcevic B, Lewellyn A, Hartley R, Roy L, Izumi T, Erikson E, Maller JL. Induction of metaphase arrest in cleaving Xenopus embryos by MAP kinase. Science. 1993;262:1262–1265. - PubMed
-
- Bhatt RR, Ferrell JE., Jr The protein kinase p90 rsk as an essential mediator of cytostatic factor activity. Science. 1999;286:1362–1365. - PubMed
-
- Gross SD, Schwab MS, Lewellyn AL, Maller JL. Induction of metaphase arrest in cleaving Xenopus embryos by the protein kinase p90Rsk. Science. 1999;286:1365–1367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

