Diverse subcellular locations of cryptogein-induced reactive oxygen species production in tobacco Bright Yellow-2 cells
- PMID: 17277088
- PMCID: PMC1851816
- DOI: 10.1104/pp.106.090902
Diverse subcellular locations of cryptogein-induced reactive oxygen species production in tobacco Bright Yellow-2 cells
Abstract
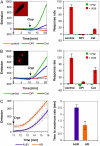
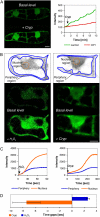
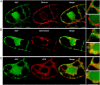
Reactive oxygen species (ROS) play a crucial role in many cellular responses and signaling pathways, including the oxidative burst defense response to pathogens. We have examined very early events in cryptogein-induced ROS production in tobacco (Nicotiana tabacum) Bright Yellow-2 suspension cells. Using Amplex Red and Amplex Ultra Red reagents, which report real-time H2O2 accumulation in cell populations, we show that the internal signal for H2O2 develops more rapidly than the external apoplastic signal. Subcellular accumulation of H2O2 was also followed in individual cells using the 2',7'-dichlorofluorescein diacetate fluorescent probe. Major accumulation was detected in endomembrane, cytoplasmic, and nuclear compartments. When cryptogein was added, the signal developed first in the nuclear region and, after a short delay, in the cell periphery. Interestingly, isolated nuclei were capable of producing H2O2 in a calcium-dependent manner, implying that nuclei can serve as a potential active source of ROS production. These results show complex spatial compartmentalization for ROS accumulation and an unexpected temporal sequence of events that occurs after cryptogein application, suggesting novel intricacy in ROS-signaling cascades.
Figures


References
-
- Barcelo AR (2005) Xylem parenchyma cells deliver the H2O2 necessary for lignification in differentiating xylem vessels. Planta 220 747–756 - PubMed
-
- Bolte S, Talbot C, Boutte Y, Catrice O, Read ND, Satiat-Jeunemaitre B (2004) FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J Microsc (Oxf) 214 159–173 - PubMed
-
- Bourque S, Binet MN, Ponchet M, Pugin A, Lebrun-Garcia A (1999) Characterization of the cryptogein binding sites on plant plasma membranes. J Biol Chem 274 34699–34705 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

