Developmental regulation of an adhesin gene during cellular morphogenesis in the fungal pathogen Candida albicans
- PMID: 17277173
- PMCID: PMC1865654
- DOI: 10.1128/EC.00340-06
Developmental regulation of an adhesin gene during cellular morphogenesis in the fungal pathogen Candida albicans
Abstract
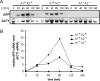
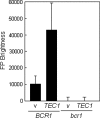
Candida albicans expresses specific virulence traits that promote disease establishment and progression. These traits include morphological transitions between yeast and hyphal growth forms that are thought to contribute to dissemination and invasion and cell surface adhesins that promote attachment to the host. Here, we describe the regulation of the adhesin gene ALS3, which is expressed specifically during hyphal development in C. albicans. Using a combination of reporter constructs and regulatory mutants, we show that this regulation is mediated by multiple factors at the transcriptional level. The analysis of ALS3 promoter deletions revealed that this promoter contains two activation regions: one is essential for activation during hyphal development, while the second increases the amplitude of this activation. Further deletion analyses using the Renilla reniformis luciferase reporter delineate the essential activation region between positions -471 and -321 of the promoter. Further 5' or 3' deletions block activation. ALS3 transcription is repressed mainly by Nrg1 and Tup1, but Rfg1 contributes to this repression. Efg1, Tec1, and Bcr1 are essential for the transcriptional activation of ALS3, with Tec1 mediating its effects indirectly through Bcr1 rather than through the putative Tec1 sites in the ALS3 promoter. ALS3 transcription is not affected by Cph2, but Cph1 contributes to full ALS3 activation. The data suggest that multiple morphogenetic signaling pathways operate through the promoter of this adhesin gene to mediate its developmental regulation in this major fungal pathogen.
Figures

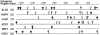

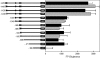

References
-
- Barelle, C. J., C. L. Manson, D. M. MacCallum, F. C. Odds, N. A. R. Gow, and A. J. P. Brown. 2004. GFP as a quantitative reporter of gene regulation in Candida albicans. Yeast 21:333-340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

