Association free energy of dipalmitoylphosphatidylserines in a mixed dipalmitoylphosphatidylcholine membrane
- PMID: 17277191
- PMCID: PMC1852338
- DOI: 10.1529/biophysj.106.089078
Association free energy of dipalmitoylphosphatidylserines in a mixed dipalmitoylphosphatidylcholine membrane
Abstract
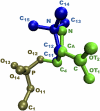
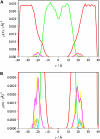
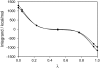
Blood coagulation is strongly dependent on the binding of vitamin K-dependent proteins to cell membranes containing phosphatidylserine (PS) via gamma-carboxyglutamic acid (Gla) domains. The process depends on calcium, which can induce nonideal behavior in membranes through domain formation. Such domain separation mediated by Ca(2+) ions or proteins can have an important contribution to the thermodynamics of the interaction between charged peripheral proteins and oppositely charged membranes. To characterize the properties of lipid-lipid interactions, molecular dynamics, and free energy simulations in a mixed bilayer membrane containing dipalmitoylphosphatidylcholine and dipalmitoylphosphatidylserine were carried out. The free energy of association between dipalmitoylphosphatidylserines in the environment of dipalmitoylphosphatidylcholines has been calculated by using a novel approach to the dual topology technique of the PS-PC hybrid. Two different methods, free energy perturbation and thermodynamic integration, were used to calculate the free energy difference. In thermodynamic integration runs three schemes were applied to evaluate the integral at the limits of lambda --> 0 or lambda --> 1. Our studies show that the association of two PSs in the environment of PCs is repulsive in the absence of Ca(2+) and becomes favorable in their presence. We also show that the mixed component membrane should exhibit nonideal behavior that will lead to PS clustering.
Figures
Similar articles
-
The PT1-Ca2+ Gla domain binds to a membrane through two dipalmitoylphosphatidylserines. A computational study.Biochemistry. 2008 Dec 16;47(50):13267-78. doi: 10.1021/bi801199v. Biochemistry. 2008. PMID: 19086158
-
Nonideal mixing of phosphatidylserine and phosphatidylcholine in the fluid lamellar phase.Biophys J. 1993 Feb;64(2):413-25. doi: 10.1016/S0006-3495(93)81382-1. Biophys J. 1993. PMID: 8457667 Free PMC article.
-
Mixed bilayer containing dipalmitoylphosphatidylcholine and dipalmitoylphosphatidylserine: lipid complexation, ion binding, and electrostatics.Biophys J. 2003 Nov;85(5):3120-31. doi: 10.1016/S0006-3495(03)74730-4. Biophys J. 2003. PMID: 14581212 Free PMC article.
-
Domain formation induced by the adsorption of charged proteins on mixed lipid membranes.Biophys J. 2005 Mar;88(3):1702-14. doi: 10.1529/biophysj.104.048132. Epub 2004 Dec 30. Biophys J. 2005. PMID: 15626713 Free PMC article.
-
Lipid modulation of protein-induced membrane domains as a mechanism for controlling signal transduction.Biochemistry. 2004 Jun 8;43(22):7102-10. doi: 10.1021/bi036334t. Biochemistry. 2004. PMID: 15170347
Cited by
-
Electrostatic field effects on membrane domain segregation and on lateral diffusion.Biophys Rev. 2011 Dec;3(4):185-192. doi: 10.1007/s12551-011-0057-4. Epub 2011 Sep 6. Biophys Rev. 2011. PMID: 28510045 Free PMC article. Review.
-
Structural basis of membrane invagination by F-BAR domains.Cell. 2008 Mar 7;132(5):807-17. doi: 10.1016/j.cell.2007.12.041. Cell. 2008. PMID: 18329367 Free PMC article.
-
A molecular dynamics investigation of lipid bilayer perturbation by PIP2.Biophys J. 2010 Jan 20;98(2):240-7. doi: 10.1016/j.bpj.2009.09.063. Biophys J. 2010. PMID: 20338845 Free PMC article.
-
The complex nature of calcium cation interactions with phospholipid bilayers.Sci Rep. 2016 Dec 1;6:38035. doi: 10.1038/srep38035. Sci Rep. 2016. PMID: 27905555 Free PMC article.
-
Alchemical Free Energy Calculations on Membrane-Associated Proteins.J Chem Theory Comput. 2023 Nov 14;19(21):7437-7458. doi: 10.1021/acs.jctc.3c00365. Epub 2023 Oct 30. J Chem Theory Comput. 2023. PMID: 37902715 Free PMC article. Review.
References
-
- Mann, K. G. 1999. Biochemistry and physiology of blood coagulation. Thromb. Haemost. 82:165–174. - PubMed
-
- Jenny, N. S., and K. Mann. G. 2003. Coagulation cascade: an overview. In Thrombosis and Hemorrhage, 3rd Ed. J. Loscazo and A. I. Schafer, editors. Lippincott Williams and Wilkins, New York. 1–21.
-
- Davie, E. W., K. Fujikawa, and W. Kisiel. 1991. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 30:10363–10370. - PubMed
-
- Jesty, J., and Y. Nemerson. 1995. The pathways of blood coagulation. In Williams Hematology, 5th Ed. E. Beutler, M. A. Lichtman, B. S. Coller, and T. J. Kipps, editors. McGraw-Hill, New York. 1227–1238.
-
- Lentz, B. R. 2003. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog. Lipid Res. 42:423–438. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

