Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands
- PMID: 17277192
- PMCID: PMC1831685
- DOI: 10.1529/biophysj.106.089730
Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands
Abstract
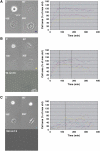
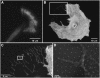
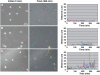
Integrin-mediated adhesion is regulated by multiple features of the adhesive surface, including its chemical composition, topography, and physical properties. In this study we investigated integrin lateral clustering, as a mechanism to control integrin functions, by characterizing the effect of nanoscale variations in the spacing between adhesive RGD ligands on cell spreading, migration, and focal adhesion dynamics. For this purpose, we used nanopatterned surfaces, containing RGD-biofunctionalized gold dots, surrounded by passivated gaps. By varying the spacing between the dots, we modulated the clustering of the associated integrins. We show that cell-surface attachment is not sensitive to pattern density, whereas the formation of stable focal adhesions and persistent spreading is. Thus cells plated on a 108-nm-spaced pattern exhibit delayed spreading with repeated protrusion-retraction cycles compared to cells growing on a 58-nm pattern. Cell motility on these surfaces is erratic and nonpersistent, leaving thin membrane tethers bound to the RGD pattern. Dynamic molecular profiling indicated that the adhesion sites formed with the 108-nm pattern undergo rapid turnover and contain reduced levels of zyxin. These findings indicate that a critical RGD density is essential for the establishment of mature and stable integrin adhesions, which, in turn, induce efficient cell spreading and formation of focal adhesions.
Figures





Similar articles
-
Lateral spacing of integrin ligands influences cell spreading and focal adhesion assembly.Eur J Cell Biol. 2006 Apr;85(3-4):219-24. doi: 10.1016/j.ejcb.2005.09.011. Epub 2005 Oct 10. Eur J Cell Biol. 2006. PMID: 16546564
-
Cell adhesion and motility depend on nanoscale RGD clustering.J Cell Sci. 2000 May;113 ( Pt 10):1677-86. doi: 10.1242/jcs.113.10.1677. J Cell Sci. 2000. PMID: 10769199
-
Early molecular events in the assembly of the focal adhesion-stress fiber complex during fibroblast spreading.Cell Motil Cytoskeleton. 2004 Jul;58(3):143-59. doi: 10.1002/cm.20005. Cell Motil Cytoskeleton. 2004. PMID: 15146534
-
The inner lives of focal adhesions.Trends Cell Biol. 2002 Aug;12(8):382-9. doi: 10.1016/s0962-8924(02)02321-8. Trends Cell Biol. 2002. PMID: 12191915 Review.
-
Integrins in cell migration.Cold Spring Harb Perspect Biol. 2011 Sep 1;3(9):a005074. doi: 10.1101/cshperspect.a005074. Cold Spring Harb Perspect Biol. 2011. PMID: 21885598 Free PMC article. Review.
Cited by
-
Cell instructive microporous scaffolds through interface engineering.J Am Chem Soc. 2012 Dec 12;134(49):20103-9. doi: 10.1021/ja308523f. Epub 2012 Nov 30. J Am Chem Soc. 2012. PMID: 23163574 Free PMC article.
-
On the energy efficiency of cell migration in diverse physical environments.Proc Natl Acad Sci U S A. 2019 Nov 26;116(48):23894-23900. doi: 10.1073/pnas.1907625116. Epub 2019 Nov 12. Proc Natl Acad Sci U S A. 2019. PMID: 31719206 Free PMC article.
-
A homing system targets therapeutic T cells to brain cancer.Nature. 2018 Sep;561(7723):331-337. doi: 10.1038/s41586-018-0499-y. Epub 2018 Sep 5. Nature. 2018. Retraction in: Nature. 2019 Mar;567(7746):132. doi: 10.1038/s41586-019-0967-z. PMID: 30185905 Free PMC article. Retracted.
-
Multi-component extracellular matrices based on peptide self-assembly.Chem Soc Rev. 2010 Sep;39(9):3413-24. doi: 10.1039/b914337h. Epub 2010 Jul 5. Chem Soc Rev. 2010. PMID: 20603663 Free PMC article. Review.
-
Peptide nucleic acid based tension sensor for cellular force imaging with strong DNase resistance.Biosens Bioelectron. 2020 Feb 15;150:111959. doi: 10.1016/j.bios.2019.111959. Epub 2019 Dec 10. Biosens Bioelectron. 2020. PMID: 31929090 Free PMC article.
References
-
- Hynes, R. O. 1987. Integrins: a family of cell surface receptors. Cell. 48:549–554. - PubMed
-
- Pavalko, F. M., and C. A. Otey. 1994. Role of adhesion molecule cytoplasmic domains in mediating interactions with the cytoskeleton. Proc. Soc. Exp. Biol. Med. 205:282–293. - PubMed
-
- Geiger, B., and A. D. Bershadsky. 2001. Transmembrane crosstalk between the extracellular matrix-cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2:793–805. - PubMed
-
- Ruoslahti, E. 1996. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12:697–715. - PubMed
-
- Yamada, K. M., and B. Geiger. 1997. Molecular interactions in cell adhesion complexes. Curr. Opin. Cell Biol. 9:76–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

