Efficient synthesis of simvastatin by use of whole-cell biocatalysis
- PMID: 17277201
- PMCID: PMC1855665
- DOI: 10.1128/AEM.02820-06
Efficient synthesis of simvastatin by use of whole-cell biocatalysis
Abstract
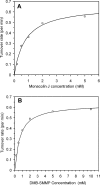
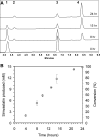
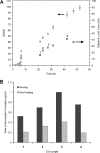
Simvastatin is a semisynthetic derivative of the fungal polyketide lovastatin and is an important drug for lowering cholesterol levels in adults. We have developed a one-step, whole-cell biocatalytic process for the synthesis of simvastatin from monacolin J. By using an Escherichia coli strain overexpressing the previously discovered acyltransferase LovD (X. Xie, K. Watanabe, W. A. Wojcicki, C. C. Wang, and Y. Tang, Chem. Biol. 13:1161-1169, 2006), we were able to achieve >99% conversion of monacolin J to simvastatin without the use of any chemical protection steps. The key finding was a membrane-permeable substrate, alpha-dimethylbutyryl-S-methyl-mercaptopropionate, that was efficiently utilized by LovD as the acyl donor. The process was scaled up for gram-scale synthesis of simvastatin. We also demonstrated that simvastatin synthesized via this method can be readily purified from the fermentation broth with >90% recovery and >98% purity as determined by high-performance liquid chromatography. Bioconversion using high-cell-density, fed-batch fermentation was also examined. The whole-cell biocatalysis can therefore be an attractive alternative to currently used multistep semisynthetic transformations.
Figures

References
-
- Alberts, A. W., J. Chen, G. Kuron, V. Hunt, J. Huff, C. Hoffman, J. Rothrock, M. Lopez, H. Joshua, E. Harris, A. Patchett, R. Monaghan, S. Currie, E. Stapley, G. Albers-Schonberg, O. Hensens, J. Hirshfield, K. Hoogsteen, J. Liesch, and J. Springer. 1980. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc. Natl. Acad. Sci. USA 77:3957-3961. - PMC - PubMed
-
- Askin, D., T. R. Verhoeven, T. M.-H. Liu, and I. Shinkai. 1991. Synthesis of synvinolin: extremely high conversion alkylation of an ester enolate. J. Org. Chem. 56:4929-4932.
-
- Endo, A. 1980. Monacolin K, a new hypocholesterolemic agent that specifically inhibits 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Antibiot. (Tokyo) 33:334-336. - PubMed
-
- Hendrickson, L., C. R. Davis, C. Roach, D. K. Nguyen, T. Aldrich, P. C. McAda, and C. D. Reeves. 1999. Lovastatin biosynthesis in Aspergillus terreus: characterization of blocked mutants, enzyme activities and a multifunctional polyketide synthase gene. Chem. Biol. 6:429-439. - PubMed
-
- Hoffman, W. F., A. W. Alberts, P. S. Anderson, J. S. Chen, R. L. Smith, and A. K. Willard. 1986. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors. 4. Side chain ester derivatives of mevinolin. J. Med. Chem. 29:849-852. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

