Nucleophosmin suppresses oncogene-induced apoptosis and senescence and enhances oncogenic cooperation in cells with genomic instability
- PMID: 17277230
- PMCID: PMC2850567
- DOI: 10.1093/carcin/bgm025
Nucleophosmin suppresses oncogene-induced apoptosis and senescence and enhances oncogenic cooperation in cells with genomic instability
Abstract
Cells from patients with genomic instability syndromes have high predisposition to cancer. However, little is known about whether these mutant cells have high susceptibility to oncogenic transformation. We have tested the hypothesis that a defect in maintaining genome integrity is necessary but not sufficient alone for oncogenic transformation and needs to collaborate with other signals in order to produce full oncogenic transformation. Using genetically matched primary cells deficient for the Fanconi complementation group C gene (Fancc) and the ataxia telangiectasia mutated gene (Atm), we found that certain forms of oncogenic activation and cooperation require a combination of genomic instability with increased expression of nucleophosmin (NPM) to prevent oncogenic stress-induced apoptosis or senescence. Intriguingly, co-expression of c-Myc and NPM leads to a synergistic increase in the proliferation rate in Fancc-/- or Atm-/- cells. Analysis of p53 stabilization and activation by c-Myc demonstrates that over-expression of NPM significantly reduces the accumulation of the activated p53 but not the stability of p53. Moreover, NPM is shown to enhance transforming activity of co-expressed Myc and Ras in wild-type and, to a greater degree, in Fancc-/- or Atm-/- cells, suggesting a role in oncogenic cooperation. Finally, a partial knockdown of NPM is sufficient to cause massive apoptosis in Fancc-/- or Atm-/- cells co-expressing c-Myc and Ras while sparing untransformed cells. Our study demonstrates a novel mechanism of NPM tumorigenesis by establishing NPM as a crucial inhibitor of oncogene-induced apoptosis and senescence.
Conflict of interest statement
Figures

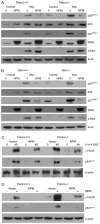
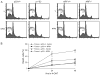

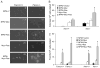
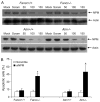
References
-
- Strasser A, et al. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990;348:331–333. - PubMed
-
- Elson A, et al. The MMTV/c-myc transgene and p53 null alleles collaborate to induce T-cell lymphomas, but not mammary carcinomas in transgenic mice. Oncogene. 1995;11:181–190. - PubMed
-
- Bringold F, et al. Tumor suppressors and oncogenes in cellular senescence. Exp Gerontol. 2000;35:317–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

