Metabolic remodeling of malignant gliomas for enhanced sensitization during radiotherapy: an in vitro study
- PMID: 17277695
- PMCID: PMC3385862
- DOI: 10.1227/01.NEU.0000249218.65332.BF
Metabolic remodeling of malignant gliomas for enhanced sensitization during radiotherapy: an in vitro study
Abstract
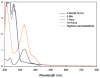
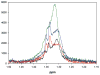
Objective: To investigate a novel method to enhance radiosensitivity of gliomas via modification of metabolite flux immediately before radiotherapy. Malignant gliomas are highly glycolytic and produce copious amounts of lactic acid, which is effluxed to the tumor microenvironment via lactate transporters. We hypothesized that inhibition of lactic acid efflux would alter glioma metabolite profiles, including those that are radioprotective. H magnetic resonance spectroscopy (MRS) was used to quantify key metabolites, including those most effective for induction of low-dose radiation-induced cell death.
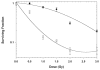
Methods: We inhibited lactate transport in U87-MG gliomas with alpha-cyano-4-hydroxycinnamic acid (ACCA). Flow cytometry was used to assess induction of cell death in treated cells. Cells were analyzed by MRS after ACCA treatment. Control and treated cells were subjected to low-dose irradiation, and the surviving fractions of cells were determined by clonogenic assays.
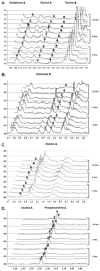
Results: MRS revealed changes to intracellular lactate on treatment with ACCA. Significant decreases in the metabolites taurine, glutamate, glutathione, alanine, and glycine were observed, along with inversion of the choline/phosphocholine profile. On exposure to low-dose radiation, ACCA-pretreated U-87MG cells underwent rapid morphological changes, which were followed by apoptotic cell death.
Conclusion: Inhibition of lactate efflux in malignant gliomas results in alterations of glycolytic metabolism, including decreased levels of the antioxidants taurine and glutathione and enhanced radiosensitivity of ACCA-treated cells. Thus, in situ application of lactate transport inhibitors such as ACCA as a novel adjunctive therapeutic strategy against glial tumors may greatly enhance the level of radiation-induced cell killing during a combined radio- and chemotherapeutic regimen.
Conflict of interest statement
None of the authors received any financial support in conjunction with the generation of this study. None of the authors has a financial conflict of interest.
Figures



References
-
- Ahmad IM, Aykin-Burns N, Sim JE, Walsh SA, Higashikubo R, Buettner GR, Venkataraman S, Mackey MA, Flanagan SW, Oberley LW, Spitz DR. Mitochondrial O2*- and H2O2 mediate glucose deprivation-induced stress in human cancer cells. J Biol Chem. 2005;280:4254–4263. - PubMed
-
- Baggetto LG. Deviant energetic metabolism of glycolytic cancer cells. Biochimie. 1992;74:959–974. - PubMed
-
- Beauchesne PD, Bertrand S, Branche R, Linke SP, Revel R, Dore JF, Pedeux RM. Human alignant glioma cell lines are sensitive to low radiation doses. Int J Cancer. 2003;105:33–40. - PubMed
-
- Berg JM, Tymoczko JL, Stryer L. Biochemistry. New York: W.H. Freeman; 2002.
-
- Brand K. Aerobic glycolysis by proliferating cells: Protection against oxidative stress at the expense of energy yield. J Bioenerg Biomembr. 1997;29:355–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

