Chelate control of diiron(I) dithiolates relevant to the [Fe-Fe]- hydrogenase active site
- PMID: 17279743
- PMCID: PMC2430879
- DOI: 10.1021/ic0618706
Chelate control of diiron(I) dithiolates relevant to the [Fe-Fe]- hydrogenase active site
Abstract
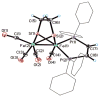
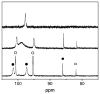
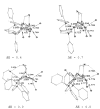
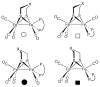
The reaction of Fe2(S2C2H4)(CO)6 with cis-Ph2PCH=CHPPh2 (dppv) yields Fe2(S2C2H4)(CO)4(dppv), 1(CO)4, wherein the dppv ligand is chelated to a single iron center. NMR analysis indicates that in 1(CO)4, the dppv ligand spans axial and basal coordination sites. In addition to the axial-basal isomer, the 1,3-propanedithiolate and azadithiolate derivatives exist as dibasal isomers. Density functional theory (DFT) calculations indicate that the axial-basal isomer is destabilized by nonbonding interactions between the dppv and the central NH or CH2 of the larger dithiolates. The Fe(CO)3 subunit in 1(CO)4 undergoes substitution with PMe3 and cyanide to afford 1(CO)3(PMe3) and (Et4N)[1(CN)(CO)3], respectively. Kinetic studies show that 1(CO)4 reacts faster with donor ligands than does its parent Fe2(S2C2H4)(CO)6. The rate of reaction of 1(CO)4 with PMe3 was first order in each reactant, k = 3.1 x 10(-4) M(-1) s(-1). The activation parameters for this substitution reaction, DeltaH = 5.8(5) kcal/mol and DeltaS = -48(2) cal/deg.mol, indicate an associative pathway. DFT calculations suggest that, relative to Fe2(S2C2H4)(CO)6, the enhanced electrophilicity of 1(CO)4 arises from the stabilization of a "rotated" transition state, which is favored by the unsymmetrically disposed donor ligands. Oxidation of MeCN solutions of 1(CO)3(PMe3) with Cp2FePF6 yielded [Fe2(S2C2H4)(mu-CO)(CO)2(dppv)(PMe3)(NCMe)](PF6)2. Reaction of this compound with PMe3 yielded [Fe2(S2C2H4)(mu-CO)(CO)(dppv)(PMe3)2(NCMe)](PF6)2.
Figures
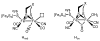
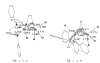
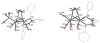

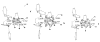


Similar articles
-
Diiron azadithiolates as models for the [FeFe]-hydrogenase active site and paradigm for the role of the second coordination sphere.Acc Chem Res. 2015 Jul 21;48(7):2107-16. doi: 10.1021/acs.accounts.5b00177. Epub 2015 Jun 16. Acc Chem Res. 2015. PMID: 26079848 Free PMC article.
-
Diferrous cyanides as models for the Fe-only hydrogenases.J Am Chem Soc. 2005 Aug 10;127(31):11010-8. doi: 10.1021/ja051584d. J Am Chem Soc. 2005. PMID: 16076208
-
Electron-rich diferrous-phosphane-thiolates relevant to Fe-only hydrogenase: is cyanide "nature's trimethylphosphane"?Chemistry. 2005 Dec 16;12(1):90-8. doi: 10.1002/chem.200500752. Chemistry. 2005. PMID: 16220561
-
Nitrosyl derivatives of diiron(I) dithiolates mimic the structure and Lewis acidity of the [FeFe]-hydrogenase active site.J Am Chem Soc. 2008 Sep 10;130(36):12021-30. doi: 10.1021/ja802268p. Epub 2008 Aug 14. J Am Chem Soc. 2008. PMID: 18700771 Free PMC article.
-
Synthetic and structural studies on [Fe2(SR)2(CN)x(CO)6-x](x-) as active site models for Fe-only hydrogenases.J Am Chem Soc. 2001 Dec 19;123(50):12518-27. doi: 10.1021/ja016071v. J Am Chem Soc. 2001. PMID: 11741415
Cited by
-
Diiron azadithiolates as models for the [FeFe]-hydrogenase active site and paradigm for the role of the second coordination sphere.Acc Chem Res. 2015 Jul 21;48(7):2107-16. doi: 10.1021/acs.accounts.5b00177. Epub 2015 Jun 16. Acc Chem Res. 2015. PMID: 26079848 Free PMC article.
-
Precursors to [FeFe]-hydrogenase models: syntheses of Fe2(SR)2(CO)6 from CO-free iron sources.Inorg Chem. 2008 Aug 4;47(15):7002-8. doi: 10.1021/ic800601k. Epub 2008 Jul 9. Inorg Chem. 2008. PMID: 18610969 Free PMC article.
-
New nitrosyl derivatives of diiron dithiolates related to the active site of the [FeFe]-hydrogenases.Inorg Chem. 2008 Dec 15;47(24):11816-24. doi: 10.1021/ic801542w. Inorg Chem. 2008. PMID: 19007207 Free PMC article.
-
Mild redox complementation enables H2 activation by [FeFe]-hydrogenase models.J Am Chem Soc. 2011 Jun 1;133(21):8098-101. doi: 10.1021/ja201731q. Epub 2011 May 6. J Am Chem Soc. 2011. PMID: 21548619 Free PMC article.
-
Synthetic models for the active site of the [FeFe]-hydrogenase: catalytic proton reduction and the structure of the doubly protonated intermediate.J Am Chem Soc. 2012 Nov 14;134(45):18843-52. doi: 10.1021/ja309216v. Epub 2012 Nov 5. J Am Chem Soc. 2012. PMID: 23126330 Free PMC article.
References
-
-
Basic Research Needs for the Hydrogen Economy. Report of the Basic Energy Sciences Workshop on Hydrogen Production, Storage, and Use.
-
-
- Linck RC, Rauchfuss TB. In: Bioorganometallics: Biomolecules, Labeling, Medicine. Jaouen G, editor. Wiley-VCH; Weinheim, Germany: 2005.
-
- Cammack R, Frey M, Robson R. Hydrogen as a Fuel: Learning from Nature. Taylor & Francis; London: 2001.
-
- Frey M. Chem Bio Chem. 2002;3:153–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

