Thio modification of yeast cytosolic tRNA is an iron-sulfur protein-dependent pathway
- PMID: 17283054
- PMCID: PMC1899921
- DOI: 10.1128/MCB.01321-06
Thio modification of yeast cytosolic tRNA is an iron-sulfur protein-dependent pathway
Abstract
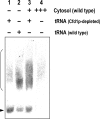
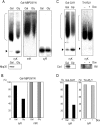
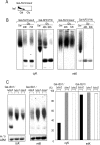
Defects in the yeast cysteine desulfurase Nfs1 cause a severe impairment in the 2-thio modification of uridine of mitochondrial tRNAs (mt-tRNAs) and cytosolic tRNAs (cy-tRNAs). Nfs1 can also provide the sulfur atoms of the iron-sulfur (Fe/S) clusters generated by the mitochondrial and cytosolic Fe/S cluster assembly machineries, termed ISC and CIA, respectively. Therefore, a key question remains as to whether the biosynthesis of Fe/S clusters is a prerequisite for the 2-thio modification of the tRNAs in both of the subcellular compartments of yeast cells. To elucidate this question, we asked whether mitochondrial ISC and/or cytosolic CIA components besides Nfs1 were involved in the 2-thio modification of these tRNAs. We demonstrate here that the three CIA components, Cfd1, Nbp35, and Cia1, are required for the 2-thio modification of cy-tRNAs but not of mt-tRNAs. Interestingly, the mitochondrial scaffold proteins Isu1 and Isu2 are required for the 2-thio modification of the cy-tRNAs but not of the mt-tRNAs, while mitochondrial Nfs1 is required for both 2-thio modifications. These results clearly indicate that the 2-thio modification of cy-tRNAs is Fe/S protein dependent and thus requires both CIA and ISC machineries but that of mt-tRNAs is Fe/S cluster independent and does not require key mitochondrial ISC components except for Nfs1.
Figures


Similar articles
-
The Cfd1-Nbp35 complex acts as a scaffold for iron-sulfur protein assembly in the yeast cytosol.Nat Chem Biol. 2007 May;3(5):278-86. doi: 10.1038/nchembio872. Epub 2007 Apr 1. Nat Chem Biol. 2007. PMID: 17401378
-
The essential WD40 protein Cia1 is involved in a late step of cytosolic and nuclear iron-sulfur protein assembly.Mol Cell Biol. 2005 Dec;25(24):10833-41. doi: 10.1128/MCB.25.24.10833-10841.2005. Mol Cell Biol. 2005. PMID: 16314508 Free PMC article.
-
How Escherichia coli and Saccharomyces cerevisiae build Fe/S proteins.Adv Microb Physiol. 2005;50:41-101. doi: 10.1016/S0065-2911(05)50002-X. Adv Microb Physiol. 2005. PMID: 16221578
-
Sulfur Modifications of the Wobble U34 in tRNAs and their Intracellular Localization in Eukaryotic Cells.Biomolecules. 2017 Feb 18;7(1):17. doi: 10.3390/biom7010017. Biomolecules. 2017. PMID: 28218716 Free PMC article. Review.
-
Mechanisms of iron-sulfur protein maturation in mitochondria, cytosol and nucleus of eukaryotes.Biochim Biophys Acta. 2006 Jul;1763(7):652-67. doi: 10.1016/j.bbamcr.2006.05.011. Epub 2006 May 23. Biochim Biophys Acta. 2006. PMID: 16843540 Review.
Cited by
-
A [3Fe-4S] cluster is required for tRNA thiolation in archaea and eukaryotes.Proc Natl Acad Sci U S A. 2016 Nov 8;113(45):12703-12708. doi: 10.1073/pnas.1615732113. Epub 2016 Oct 24. Proc Natl Acad Sci U S A. 2016. PMID: 27791189 Free PMC article.
-
Dual role of the molybdenum cofactor biosynthesis protein MOCS3 in tRNA thiolation and molybdenum cofactor biosynthesis in humans.J Biol Chem. 2012 May 18;287(21):17297-17307. doi: 10.1074/jbc.M112.351429. Epub 2012 Mar 27. J Biol Chem. 2012. PMID: 22453920 Free PMC article.
-
Roles of Elongator Dependent tRNA Modification Pathways in Neurodegeneration and Cancer.Genes (Basel). 2018 Dec 28;10(1):19. doi: 10.3390/genes10010019. Genes (Basel). 2018. PMID: 30597914 Free PMC article. Review.
-
Recent Advances in Our Understanding of the Biosynthesis of Sulfur Modifications in tRNAs.Front Microbiol. 2018 Nov 1;9:2679. doi: 10.3389/fmicb.2018.02679. eCollection 2018. Front Microbiol. 2018. PMID: 30450093 Free PMC article.
-
Characterization and interaction studies of two isoforms of the dual localized 3-mercaptopyruvate sulfurtransferase TUM1 from humans.J Biol Chem. 2014 Dec 12;289(50):34543-56. doi: 10.1074/jbc.M114.605733. Epub 2014 Oct 21. J Biol Chem. 2014. PMID: 25336638 Free PMC article.
References
-
- Dong, J., R. Lai, K. Nielsen, C. A. Fekete, H. Qiu, and A. G. Hinnebusch. 2004. The essential ATP-binding cassette protein RLI1 functions in translation by promoting preinitiation complex assembly. J. Biol. Chem. 279:42157-42168. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
