Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia
- PMID: 17283055
- PMCID: PMC1899915
- DOI: 10.1128/MCB.00072-07
Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia
Abstract
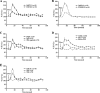
Recent evidence suggests that fibrocystin/polyductin (FPC), polycystin-1 (PC1), and polycystin-2 (PC2) are all localized at the plasma membrane and the primary cilium, where PC1 and PC2 contribute to fluid flow sensation and may function in the same mechanotransduction pathways. To further define the exact subcellular localization of FPC, the protein product encoded by the PKHD1 gene responsible for autosomal recessive polycystic kidney disease (PKD) in humans, and whether FPC has direct and/or indirect cross talk with PC2, which, in turn, is pivotal for the pathogenesis of autosomal dominant PKD, we performed double immunostaining and coimmunoprecipitation as well as a microfluorimetry study of kidney tubular epithelial cells. FPC and PC2 are found to completely or partially colocalize at the plasma membrane and the primary cilium and can be reciprocally coimmunoprecipitated. Although incomplete removal of FPC by small interfering RNA and antibody 803 to intracellular epitopes of FPC did not abolish flow-induced intracellular calcium responses, antibody 804 to extracellular epitopes of FPC blocked cellular calcium responses to flow stimulation. These findings suggest that FPC and polycystins share, at least in part, a common mechanotransduction pathway.
Figures







References
-
- Bergmann, C., F. Kupper, C. Dornia, F. Schneider, J. Senderek, and K. Zerres. 2005. Algorithm for efficient PKHD1 mutation screening in autosomal recessive polycystic kidney disease (ARPKD). Hum. Mutat. 25:225-231. - PubMed
-
- Reference deleted.
-
- Cai, Y., Y. Maeda, A. Cedzich, V. E. Torres, G. Wu, T. Hayashi, T. Mochizuki, J. H. Park, R. Witzgall, and S. Somlo. 1999. Identification and characterization of polycystin-2, the PKD2 gene product. J. Biol. Chem. 274:28557-28565. - PubMed
-
- Calvet, J. P., and J. J. Grantham. 2001. The genetics and physiology of polycystic kidney disease. Semin. Nephrol. 21:107-123. - PubMed
-
- Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
