The p85alpha regulatory subunit of phosphoinositide 3-kinase potentiates c-Jun N-terminal kinase-mediated insulin resistance
- PMID: 17283057
- PMCID: PMC1899914
- DOI: 10.1128/MCB.00079-07
The p85alpha regulatory subunit of phosphoinositide 3-kinase potentiates c-Jun N-terminal kinase-mediated insulin resistance
Abstract
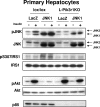
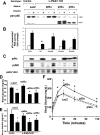
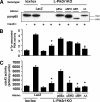
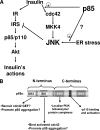
Insulin resistance is a defining feature of type 2 diabetes and the metabolic syndrome. While the molecular mechanisms of insulin resistance are multiple, recent evidence suggests that attenuation of insulin signaling by c-Jun N-terminal kinase (JNK) may be a central part of the pathobiology of insulin resistance. Here we demonstrate that the p85alpha regulatory subunit of phosphoinositide 3-kinase (PI3K), a key mediator of insulin's metabolic actions, is also required for the activation of JNK in states of insulin resistance, including high-fat diet-induced obesity and JNK1 overexpression. The requirement of the p85alpha regulatory subunit for JNK occurs independently of its role as a component of the PI3K heterodimer and occurs only in response to specific stimuli, namely, insulin and tunicamycin, a chemical that induces endoplasmic reticulum stress. We further show that insulin and p85 activate JNK by via cdc42 and MKK4. The activation of this cdc42/JNK pathway requires both an intact N terminus and functional SH2 domains within the C terminus of the p85alpha regulatory subunit. Thus, p85alpha plays a dual role in regulating insulin sensitivity and may mediate cross talk between the PI3K and stress kinase pathways.
Figures




Similar articles
-
Increased P85alpha is a potent negative regulator of skeletal muscle insulin signaling and induces in vivo insulin resistance associated with growth hormone excess.J Biol Chem. 2005 Nov 11;280(45):37489-94. doi: 10.1074/jbc.M506967200. Epub 2005 Sep 8. J Biol Chem. 2005. PMID: 16166093
-
Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria.J Biol Chem. 2009 May 29;284(22):14809-18. doi: 10.1074/jbc.M901488200. Epub 2009 Mar 30. J Biol Chem. 2009. PMID: 19332540 Free PMC article.
-
Reduced expression of the murine p85alpha subunit of phosphoinositide 3-kinase improves insulin signaling and ameliorates diabetes.J Clin Invest. 2002 Jan;109(1):141-9. doi: 10.1172/JCI13305. J Clin Invest. 2002. PMID: 11781359 Free PMC article.
-
Inflammation and endoplasmic reticulum stress in obesity and diabetes.Int J Obes (Lond). 2008 Dec;32 Suppl 7(Suppl 7):S52-4. doi: 10.1038/ijo.2008.238. Int J Obes (Lond). 2008. PMID: 19136991 Free PMC article. Review.
-
Role of oxidative stress, endoplasmic reticulum stress, and c-Jun N-terminal kinase in pancreatic beta-cell dysfunction and insulin resistance.Int J Biochem Cell Biol. 2006;38(5-6):782-93. doi: 10.1016/j.biocel.2006.01.004. Int J Biochem Cell Biol. 2006. PMID: 16607699 Review.
Cited by
-
Unique and redundant roles of class IA PI3Kinase regulatory subunits in mast cell development.Cell Cycle. 2013 Jan 1;12(1):7-8. doi: 10.4161/cc.23070. Epub 2012 Dec 19. Cell Cycle. 2013. PMID: 23255115 Free PMC article. No abstract available.
-
Class IA PI3K regulatory subunits: p110-independent roles and structures.Biochem Soc Trans. 2020 Aug 28;48(4):1397-1417. doi: 10.1042/BST20190845. Biochem Soc Trans. 2020. PMID: 32677674 Free PMC article. Review.
-
Targeting the CNS to treat type 2 diabetes.Nat Rev Drug Discov. 2009 May;8(5):386-98. doi: 10.1038/nrd2874. Nat Rev Drug Discov. 2009. PMID: 19404312 Review.
-
PKCδ regulates hepatic insulin sensitivity and hepatosteatosis in mice and humans.J Clin Invest. 2011 Jun;121(6):2504-17. doi: 10.1172/JCI46045. Epub 2011 May 16. J Clin Invest. 2011. PMID: 21576825 Free PMC article.
-
Insulin regulates TRB3 and other stress-responsive gene expression through induction of C/EBPbeta.Mol Endocrinol. 2009 Apr;23(4):475-85. doi: 10.1210/me.2008-0284. Epub 2009 Jan 22. Mol Endocrinol. 2009. PMID: 19164449 Free PMC article.
References
-
- Bandyopadhyay, G. K., J. G. Yu, J. Ofrecio, and J. M. Olefsky. 2005. Increased p85/55/50 expression and decreased phosphatidylinositol 3-kinase activity in insulin-resistant human skeletal muscle. Diabetes 54:2351-2359. - PubMed
-
- Beeton, C. A., P. Das, M. D. Waterfield, and P. R. Shepherd. 1999. The SH3 and BH domains of the p85α adapter subunit play a critical role in regulating class Ia phosphoinositide 3-kinase function. Mol. Cell. Biol. Res. Commun. 1:153-157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
