Dependence of macrophage phagocytic efficacy on antibody concentration
- PMID: 17283107
- PMCID: PMC1865677
- DOI: 10.1128/IAI.01258-06
Dependence of macrophage phagocytic efficacy on antibody concentration
Abstract
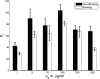
Macrophages ingest the fungus Cryptococcus neoformans only in the presence of opsonins, and this provides a remarkably clean system for the detailed analysis of phagocytosis. This system is also unusual in that antibody-mediated phagocytosis involves ingestion through both Fc and complement receptors in the absence of complement. Mathematical modeling was used to analyze and explain the experimental data that the macrophage phagocytic index increased with increasing doses of antibody despite saturating concentrations and declined at high concentrations. A model was developed that explains the increase in phagocytic index with increasing antibody doses, differentiates among the contributions from Fc and complement receptors, and provides a tool for estimating antibody concentrations that optimize efficacy of phagocytosis. Experimental results and model calculations revealed that blocking of Fc receptors by excess antibody caused a reduction in phagocytic index but increased phagocytosis through complement receptors rapidly compensated for this effect. At high antibody concentrations, a further reduction in phagocytic index was caused by interference with complement receptor ingestion as a consequence of saturation of the fungal capsule. The ability of our model to predict the antibody dose dependence of the macrophage phagocytic efficacy for C. neoformans strongly suggest that the major variables that determine the efficacy of this process have been identified. The model predicts that the affinity constant of the opsonic antibody for the Fc receptor and the association-dissociation constant of antibody from the microbial antigen are critical parameters determining the efficacy of phagocytosis.
Figures
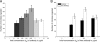




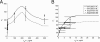
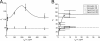
Similar articles
-
Roles of macrophage Fc and C3b receptors in phagocytosis of immunologically coated Cryptococcus neoformans.Proc Natl Acad Sci U S A. 1981 Jun;78(6):3853-7. doi: 10.1073/pnas.78.6.3853. Proc Natl Acad Sci U S A. 1981. PMID: 7022456 Free PMC article.
-
Fc- and complement-receptor activation stimulates cell cycle progression of macrophage cells from G1 to S.J Immunol. 2005 Jun 1;174(11):7226-33. doi: 10.4049/jimmunol.174.11.7226. J Immunol. 2005. PMID: 15905568
-
Macrophage hydrogen peroxide production and phagocytic function are decreased following phagocytosis mediated by Fc receptors but not complement receptors.Biochem Biophys Res Commun. 1991 Oct 15;180(1):268-72. doi: 10.1016/s0006-291x(05)81287-2. Biochem Biophys Res Commun. 1991. PMID: 1930224
-
Factors influencing the endocytosis of immune complexes.Adv Nephrol Necker Hosp. 1984;13:341-67. Adv Nephrol Necker Hosp. 1984. PMID: 6236677 Review.
-
Activation of macrophage complement receptors for phagocytosis.Contemp Top Immunobiol. 1984;13:57-70. doi: 10.1007/978-1-4757-1445-6_3. Contemp Top Immunobiol. 1984. PMID: 6375955 Review.
Cited by
-
Cryptococcus neoformans Infection in the Central Nervous System: The Battle between Host and Pathogen.J Fungi (Basel). 2022 Oct 12;8(10):1069. doi: 10.3390/jof8101069. J Fungi (Basel). 2022. PMID: 36294634 Free PMC article. Review.
-
A comprehensive mathematical model for three-body binding equilibria.J Am Chem Soc. 2013 Apr 24;135(16):6092-9. doi: 10.1021/ja311795d. Epub 2013 Apr 16. J Am Chem Soc. 2013. PMID: 23544844 Free PMC article.
-
HIV/AIDS Vaccines: 2018.Clin Pharmacol Ther. 2018 Dec;104(6):1062-1073. doi: 10.1002/cpt.1208. Epub 2018 Oct 9. Clin Pharmacol Ther. 2018. PMID: 30099743 Free PMC article. Review.
-
Salmonella Typhi Vi capsule prime-boost vaccination induces convergent and functional antibody responses.Sci Immunol. 2021 Oct 29;6(64):eabj1181. doi: 10.1126/sciimmunol.abj1181. Epub 2021 Oct 29. Sci Immunol. 2021. PMID: 34714686 Free PMC article.
-
Dynamics of Cryptococcus neoformans-macrophage interactions reveal that fungal background influences outcome during cryptococcal meningoencephalitis in humans.mBio. 2011 Aug 9;2(4):e00158-11. doi: 10.1128/mBio.00158-11. Print 2011. mBio. 2011. PMID: 21828220 Free PMC article.
References
-
- Camner, P., M. Lundborg, L. Lastbom, P. Gerde, N. Gross, and C. Jarstrand. 2002. Experimental and calculated parameters on particle phagocytosis by alveolar macrophages. J. Appl. Physiol. 92:2608-2616. - PubMed
-
- Casadevall, A. 2004. The methodology for determining the efficacy of antibody-mediated immunity. J. Immunol. Methods 291:1-10. - PubMed
-
- Casadevall, A., W. Cleare, M. Feldmesser, A. Glatman-Freedman, D. L. Goldman, T. R. Kozel, N. Lendvai, J. Mukherjee, L. Pirofski, J. Rivera, A. L. Rosas, M. D. Scharff, P. Valadon, K. Westin, and Z. Zhong. 1998. Characterization of a murine monoclonal antibody to Cryptocococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 42:1437-1446. - PMC - PubMed
-
- Dadachova, E., R. A. Bryan, C. Apostolidis, A. Morgenstern, T. Zhang, T. Moadel, M. Torres, X. Huang, E. Revskaya, and A. Casadevall. 2006. Interaction of radiolabeled antibodies with fungal cells and components of the immune system in vitro and during radioimmunotherapy for experimental fungal infection. J. Infect. Dis. 193:1427-1436. - PubMed
-
- Larsen, R. A., P. G. Pappas, J. R. Perfect, J. A. Aberg, A. Casadevall, G. A. Cloud, R. James, S. Filler, and W. E. Dismukes. 2005. A phase I evaluation of the safety and pharmacodynamic activity of a murine-derived monoclonal antibody 18b7 in subjects with treated cryptococcal meningitis. Antimicrob. Agents Chemother 49:952-958. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

