Formation of microtubule-based traps controls the sorting and concentration of vesicles to restricted sites of regenerating neurons after axotomy
- PMID: 17283182
- PMCID: PMC2063984
- DOI: 10.1083/jcb.200607098
Formation of microtubule-based traps controls the sorting and concentration of vesicles to restricted sites of regenerating neurons after axotomy
Abstract
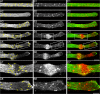
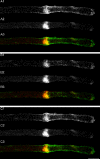
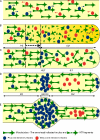
Transformation of a transected axonal tip into a growth cone (GC) is a critical step in the cascade leading to neuronal regeneration. Critical to the regrowth is the supply and concentration of vesicles at restricted sites along the cut axon. The mechanisms underlying these processes are largely unknown. Using online confocal imaging of transected, cultured Aplysia californica neurons, we report that axotomy leads to reorientation of the microtubule (MT) polarities and formation of two distinct MT-based vesicle traps at the cut axonal end. Approximately 100 microm proximal to the cut end, a selective trap for anterogradely transported vesicles is formed, which is the plus end trap. Distally, a minus end trap is formed that exclusively captures retrogradely transported vesicles. The concentration of anterogradely transported vesicles in the former trap optimizes the formation of a GC after axotomy.
Figures





Similar articles
-
Local calcium-dependent mechanisms determine whether a cut axonal end assembles a retarded endbulb or competent growth cone.Exp Neurol. 2009 Sep;219(1):112-25. doi: 10.1016/j.expneurol.2009.05.004. Epub 2009 May 13. Exp Neurol. 2009. PMID: 19442660
-
Local self-assembly mechanisms underlie the differential transformation of the proximal and distal cut axonal ends into functional and aberrant growth cones.J Comp Neurol. 2008 Mar 1;507(1):1019-30. doi: 10.1002/cne.21522. J Comp Neurol. 2008. PMID: 18092341
-
On-line confocal imaging of the events leading to structural dedifferentiation of an axonal segment into a growth cone after axotomy.J Comp Neurol. 2006 Feb 10;494(5):705-20. doi: 10.1002/cne.20690. J Comp Neurol. 2006. PMID: 16374810
-
Calcium, protease activation, and cytoskeleton remodeling underlie growth cone formation and neuronal regeneration.Cell Mol Neurobiol. 2001 Dec;21(6):591-604. doi: 10.1023/a:1015135617557. Cell Mol Neurobiol. 2001. PMID: 12043835 Free PMC article. Review.
-
Microtubule transport in the axon: Re-thinking a potential role for the actin cytoskeleton.Neuroscientist. 2006 Apr;12(2):107-18. doi: 10.1177/1073858405283428. Neuroscientist. 2006. PMID: 16514008 Review.
Cited by
-
Global up-regulation of microtubule dynamics and polarity reversal during regeneration of an axon from a dendrite.Mol Biol Cell. 2010 Mar 1;21(5):767-77. doi: 10.1091/mbc.e09-11-0967. Epub 2010 Jan 6. Mol Biol Cell. 2010. PMID: 20053676 Free PMC article.
-
Axon regeneration mechanisms: insights from C. elegans.Trends Cell Biol. 2011 Oct;21(10):577-84. doi: 10.1016/j.tcb.2011.08.003. Epub 2011 Sep 8. Trends Cell Biol. 2011. PMID: 21907582 Free PMC article. Review.
-
No simpler than mammals: axon and dendrite regeneration in Drosophila.Genes Dev. 2012 Jul 15;26(14):1509-14. doi: 10.1101/gad.198150.112. Genes Dev. 2012. PMID: 22802524 Free PMC article.
-
Normal spastin gene dosage is specifically required for axon regeneration.Cell Rep. 2012 Nov 29;2(5):1340-50. doi: 10.1016/j.celrep.2012.09.032. Epub 2012 Nov 1. Cell Rep. 2012. PMID: 23122959 Free PMC article.
-
Roles of membrane trafficking in nerve repair and regeneration.Commun Integr Biol. 2010 May;3(3):209-14. doi: 10.4161/cib.3.3.11555. Commun Integr Biol. 2010. PMID: 20714395 Free PMC article.
References
-
- Ahmad, F.J., Y. He, K.A. Myers, T.P. Hasaka, F. Francis, M.M. Black, and P.W. Baas. 2006. Effects of dynactin disruption and dynein depletion on axonal microtubules. Traffic. 7:524–537. - PubMed
-
- Akhmanova, A., and C.C. Hoogenraad. 2005. Microtubule plus-end-tracking proteins: mechanisms and functions. Curr. Opin. Cell Biol. 17:47–54. - PubMed
-
- Allan, V., and I.S. Nathke. 2001. Catch and pull a microtubule: getting a grasp on the cortex. Nat. Cell Biol. 3:E226–E228. - PubMed
-
- Ashery, U., R. Penner, and M.E. Spira. 1996. Acceleration of membrane recycling by axotomy of cultured Aplysia neurons. Neuron. 16:641–651. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

