Midbody and primary cilium of neural progenitors release extracellular membrane particles enriched in the stem cell marker prominin-1
- PMID: 17283184
- PMCID: PMC2063983
- DOI: 10.1083/jcb.200608137
Midbody and primary cilium of neural progenitors release extracellular membrane particles enriched in the stem cell marker prominin-1
Abstract
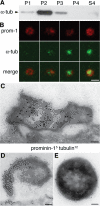
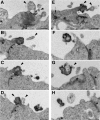
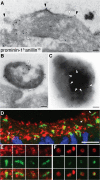
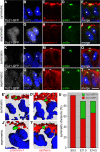
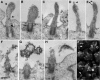
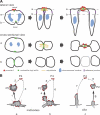
Expansion of the neocortex requires symmetric divisions of neuroepithelial cells, the primary progenitor cells of the developing mammalian central nervous system. Symmetrically dividing neuroepithelial cells are known to form a midbody at their apical (rather than lateral) surface. We show that apical midbodies of neuroepithelial cells concentrate prominin-1 (CD133), a somatic stem cell marker and defining constituent of a specific plasma membrane microdomain. Moreover, these apical midbodies are released, as a whole or in part, into the extracellular space, yielding the prominin-1-enriched membrane particles found in the neural tube fluid. The primary cilium of neuroepithelial cells also concentrates prominin-1 and appears to be a second source of the prominin-1-bearing extracellular membrane particles. Our data reveal novel origins of extracellular membrane traffic that enable neural stem and progenitor cells to avoid the asymmetric inheritance of the midbody observed for other cells and, by releasing a stem cell membrane microdomain, to potentially influence the balance of their proliferation versus differentiation.
Figures


References
-
- Aaku-Saraste, E., B. Oback, A. Hellwig, and W.B. Huttner. 1997. Neuroepithelial cells downregulate their plasma membrane polarity prior to neural tube closure and neurogenesis. Mech. Dev. 69:71–81. - PubMed
-
- Bancroft, M., and R. Bellairs. 1975. Differentiation of the neural plate and neural tube in the young chick embryo. A study by scanning and transmission electron microscopy. Anat. Embryol. (Berl.). 147:309–335. - PubMed
-
- Beaun, A.R., and G. Grondin. 1991. Shedding of vesicular material from the cell surface of eukaryotic cells: different cellular phenomena. Biochim. Biophys. Acta. 1071:203–219. - PubMed
-
- Bellairs, R., and M. Bancroft. 1975. Midbodies and beaded threads. Am. J. Anat. 143:393–398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

