A conserved surface on Toll-like receptor 5 recognizes bacterial flagellin
- PMID: 17283206
- PMCID: PMC2118731
- DOI: 10.1084/jem.20061400
A conserved surface on Toll-like receptor 5 recognizes bacterial flagellin
Abstract
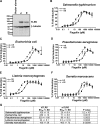
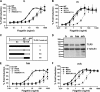
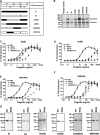
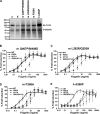
The molecular basis for Toll-like receptor (TLR) recognition of microbial ligands is unknown. We demonstrate that mouse and human TLR5 discriminate between different flagellins, and we use this difference to map the flagellin recognition site on TLR5 to 228 amino acids of the extracellular domain. Through molecular modeling of the TLR5 ectodomain, we identify two conserved surface-exposed regions. Mutagenesis studies demonstrate that naturally occurring amino acid variation in TLR5 residue 268 is responsible for human and mouse discrimination between flagellin molecules. Mutations within one conserved surface identify residues D295 and D367 as important for flagellin recognition. These studies localize flagellin recognition to a conserved surface on the modeled TLR5 structure, providing detailed analysis of the interaction of a TLR with its ligand. These findings suggest that ligand binding at the beta sheets results in TLR activation and provide a new framework for understanding TLR-agonist interactions.
Figures

References
-
- Janeway, C.A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197–216. - PubMed
-
- Medzhitov, R., and C.A. Janeway Jr. 1997. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 91:295–298. - PubMed
-
- Hayashi, F., K.D. Smith, A. Ozinsky, T.R. Hawn, E.C. Yi, D.R. Goodlett, J.K. Eng, S. Akira, D.M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 410:1099–1103. - PubMed
-
- Harshey, R.M., and A. Toguchi. 1996. Spinning tails: homologies among bacterial flagellar systems. Trends Microbiol. 4:226–231. - PubMed
-
- Smith, K.D., E. Andersen-Nissen, F. Hayashi, K. Strobe, M.A. Bergman, S.L. Barrett, B.T. Cookson, and A. Aderem. 2003. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 4:1247–1253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

