Conventional T-bet(+)Foxp3(-) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection
- PMID: 17283209
- PMCID: PMC2118735
- DOI: 10.1084/jem.20062175
Conventional T-bet(+)Foxp3(-) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection
Abstract
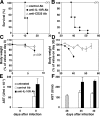
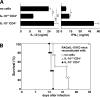
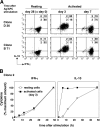
Although interferon gamma (IFN-gamma) secretion is essential for control of most intracellular pathogens, host survival often also depends on the expression of interleukin 10 (IL-10), a cytokine known to counteract IFN-gamma effector functions. We analyzed the source of regulatory IL-10 in mice infected with the protozoan parasite Toxoplasma gondii. Unexpectedly, IFN-gamma-secreting T-bet(+)Foxp3(-) T helper type 1 (Th1) cells were found to be the major producers of IL-10 in these animals. Further analysis revealed that the same IL-10(+)IFN-gamma(gamma) population displayed potent effector function against the parasite while, paradoxically, also inducing profound suppression of IL-12 production by antigen-presenting cells. Although at any given time point only a fraction of the cells appeared to simultaneously produce IL-10 and IFN-gamma, IL-10 production could be stimulated in IL-10(-)IFN-gamma(+) cells by further activation in vitro. In addition, experiments with T. gondii-specific IL-10(+)IFN-gamma(+) CD4 clones revealed that although IFN-gamma expression is imprinted and triggered with similar kinetics regardless of the state of Th1 cell activation, IL-10 secretion is induced more rapidly from recently activated than from resting cells. These findings indicate that IL-10 production by CD4(+) T lymphocytes need not involve a distinct regulatory Th cell subset but can be generated in Th1 cells as part of the effector response to intracellular pathogens.
Figures
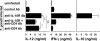




References
-
- Gajewski, T.F., and F.W. Fitch. 1988. Anti-proliferative effect of IFN-γ in immune regulation. I. IFN-γ inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J. Immunol. 140:4245–4252. - PubMed
-
- Abbas, A.K., K.M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature. 383:787–793. - PubMed
-
- Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151–1164. - PubMed
-
- Kretschmer, K., I. Apostolou, D. Hawiger, K. Khazaie, M.C. Nussenzweig, and H. von Boehmer. 2005. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 6:1219–1227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

