Targeted cell killing by reconstituted caspases
- PMID: 17283333
- PMCID: PMC1892955
- DOI: 10.1073/pnas.0610877104
Targeted cell killing by reconstituted caspases
Erratum in
- Proc Natl Acad Sci U S A. 2008 Jan 15;105(2):824
Abstract
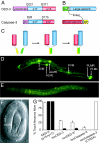
We have developed a two-component system involving reconstituted caspase (recCaspase) for selective and/or conditional ablation of targeted cells. Caspases, the executioners of programmed cell death, are normally synthesized as inactive zymogens and are activated by proteolytic processing of their subunits. We show here, using two different caspases, Caenorhabditis elegans CED-3 and human Caspase-3, that coexpression of the subunits generates constitutively active caspase activity that leads to cell death. This recCaspase activity, however, occurred only when the subunits associated through binding of linked antiparallel leucine-zipper domains. We exploited the dual-component nature of recCaspases by expressing the individual subunits from combinations of promoters either to target selectively the subset of cells for apoptosis or induce cell death in specific cells at specific times during development. The high degree of target specificity and tight regulation of induction of recCaspase would be advantageous in creating animal models that are ablated for specific cells and in other targeted cell killings.
Conflict of interest statement
Conflict of interest statement: D.S.C. has submitted a patent based on this work.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

