The spatial distribution of inositol 1,4,5-trisphosphate receptor isoforms shapes Ca2+ waves
- PMID: 17284437
- PMCID: PMC2825872
- DOI: 10.1074/jbc.M700746200
The spatial distribution of inositol 1,4,5-trisphosphate receptor isoforms shapes Ca2+ waves
Abstract
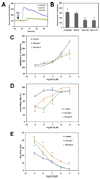
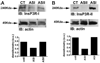
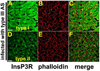
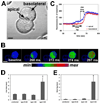
Cytosolic Ca(2+) is a versatile second messenger that can regulate multiple cellular processes simultaneously. This is accomplished in part through Ca(2+) waves and other spatial patterns of Ca(2+) signals. To investigate the mechanism responsible for the formation of Ca(2+) waves, we examined the role of inositol 1,4,5-trisphosphate receptor (InsP3R) isoforms in Ca(2+) wave formation. Ca(2+) signals were examined in hepatocytes, which express the type I and II InsP3R in a polarized fashion, and in AR4-2J cells, a nonpolarized cell line that expresses type I and II InsP3R in a ratio similar to what is found in hepatocytes but homogeneously throughout the cell. Expression of type I or II InsP3R was selectively suppressed by isoform-specific DNA antisense in an adenoviral delivery system, which was delivered to AR4-2J cells in culture and to hepatocytes in vivo. Loss of either isoform inhibited Ca(2+) signals to a similar extent in AR4-2J cells. In contrast, loss of the basolateral type I InsP3R decreased the sensitivity of hepatocytes to vasopressin but had little effect on the initiation or spread of Ca(2+) waves across hepatocytes. Loss of the apical type II isoform caused an even greater decrease in the sensitivity of hepatocytes to vasopressin and resulted in Ca(2+) waves that were much slower and delayed in onset. These findings provide evidence that the apical concentration of type II InsP3Rs is essential for the formation of Ca(2+) waves in hepatocytes. The subcellular distribution of InsP3R isoforms may critically determine the repertoire of spatial patterns of Ca(2+) signals.
Figures






Similar articles
-
Lipid rafts establish calcium waves in hepatocytes.Gastroenterology. 2007 Jul;133(1):256-67. doi: 10.1053/j.gastro.2007.03.115. Epub 2007 Apr 11. Gastroenterology. 2007. PMID: 17631147 Free PMC article.
-
The type II inositol 1,4,5-trisphosphate receptor can trigger Ca2+ waves in rat hepatocytes.Gastroenterology. 2002 Apr;122(4):1088-100. doi: 10.1053/gast.2002.32363. Gastroenterology. 2002. PMID: 11910359
-
Regulation by Ca2+ and inositol 1,4,5-trisphosphate (InsP3) of single recombinant type 3 InsP3 receptor channels. Ca2+ activation uniquely distinguishes types 1 and 3 insp3 receptors.J Gen Physiol. 2001 May;117(5):435-46. doi: 10.1085/jgp.117.5.435. J Gen Physiol. 2001. PMID: 11331354 Free PMC article.
-
[Molecular and functional diversity of inositol triphosphate-induced Ca(2+) release].Verh K Acad Geneeskd Belg. 1995;57(5):423-58. Verh K Acad Geneeskd Belg. 1995. PMID: 8571671 Review. Dutch.
-
The inositol 1,4,5-trisphosphate receptors.Cell Calcium. 2005 Sep-Oct;38(3-4):261-72. doi: 10.1016/j.ceca.2005.06.030. Cell Calcium. 2005. PMID: 16102823 Review.
Cited by
-
Transcellular calcium transport in mammary epithelial cells.J Mammary Gland Biol Neoplasia. 2007 Dec;12(4):223-35. doi: 10.1007/s10911-007-9057-1. Epub 2007 Nov 13. J Mammary Gland Biol Neoplasia. 2007. PMID: 17999165
-
Spatial-temporal patterning of Ca2+ signals by the subcellular distribution of IP3 and IP3 receptors.Semin Cell Dev Biol. 2019 Oct;94:3-10. doi: 10.1016/j.semcdb.2019.01.012. Epub 2019 Feb 2. Semin Cell Dev Biol. 2019. PMID: 30703557 Free PMC article. Review.
-
Lipid rafts establish calcium waves in hepatocytes.Gastroenterology. 2007 Jul;133(1):256-67. doi: 10.1053/j.gastro.2007.03.115. Epub 2007 Apr 11. Gastroenterology. 2007. PMID: 17631147 Free PMC article.
-
Calcium signalling and liver regeneration.Int J Hepatol. 2012;2012:630670. doi: 10.1155/2012/630670. Epub 2012 Oct 16. Int J Hepatol. 2012. PMID: 23119169 Free PMC article.
-
Nuclear Factor, Erythroid 2-Like 2 Regulates Expression of Type 3 Inositol 1,4,5-Trisphosphate Receptor and Calcium Signaling in Cholangiocytes.Gastroenterology. 2015 Jul;149(1):211-222.e10. doi: 10.1053/j.gastro.2015.03.014. Epub 2015 Mar 18. Gastroenterology. 2015. PMID: 25796361 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

