Wound-induced ATP release and EGF receptor activation in epithelial cells
- PMID: 17284517
- PMCID: PMC1853294
- DOI: 10.1242/jcs.03389
Wound-induced ATP release and EGF receptor activation in epithelial cells
Abstract
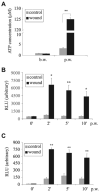
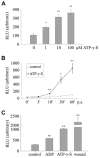
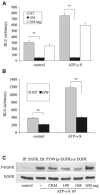
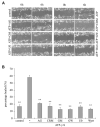
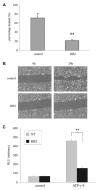
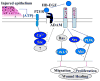
We have shown previously that wounding of human corneal epithelial (HCE) cells resulted in epidermal growth factor receptor (EGFR) transactivation through ectodomain shedding of heparin-binding EGF-like growth factor (HB-EGF). However, the initial signal to trigger these signaling events in response to cell injury remains elusive. In the present study, we investigated the role of ATP released from the injured cells in EGFR transactivation in HCE cells as well as in BEAS 2B cells, a bronchial epithelial cell line. Wounding of epithelial monolayer resulted in the release of ATP into the culture medium. The wound-induced rapid activation of phosphatidylinositol-3-kinase (PI3K) and extracellular signal-regulated kinase (ERK) pathways in HCE cells was attenuated by eliminating extracellular ATP, ADP and adenosine. The nonhydrolyzable ATP analog ATP-gamma-S induced rapid and sustained EGFR activation that depended on HB-EGF shedding and ADAM (a disintegrin and metalloproteinase). Targeting pathways leading to HB-EGF shedding and EGFR activation attenuated ATP-gamma-S-enhanced closure of small scratch wounds. The purinoceptor antagonist reactive blue 2 decreased wound closure and attenuated ATP-gamma-S induced HB-EGF shedding. Taken together, our data suggest that ATP, released upon epithelial injury, acts as an early signal to trigger cell responses including an increase in HB-EGF shedding, subsequent EGFR transactivation and its downstream signaling, resulting in wound healing.
Figures



References
-
- Agteresch HJ, Dagnelie PC, van der Gaast A, Stijnen T, Wilson JH. Randomized clinical trial of adenosine 5′-triphosphate in patients with advanced non-small-cell lung cancer. J Natl Cancer Inst. 2000;92:321–328. - PubMed
-
- Agteresch HJ, Rietveld T, Kerkhofs LG, van den Berg JW, Wilson JH, Dagnelie PC. Beneficial effects of adenosine triphosphate on nutritional status in advanced lung cancer patients: a randomized clinical trial. J Clin Oncol. 2002;20:371–378. - PubMed
-
- Ahmad S, Ahmad A, McConville G, Schneider BK, Allen CB, Manzer R, Mason RJ, White CW. Lung epithelial cells release ATP during ozone exposure: signaling for cell survival. Free Radic Biol Med. 2005;39:213–226. - PubMed
-
- Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, Handa H. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36:614–621. - PubMed
-
- Blobel CP. Functional and biochemical characterization of ADAMs and their predicted role in protein ectodomain shedding. Inflamm Res. 2002;51:83–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

