DC-HIL is a negative regulator of T lymphocyte activation
- PMID: 17284525
- PMCID: PMC1885497
- DOI: 10.1182/blood-2006-11-053769
DC-HIL is a negative regulator of T lymphocyte activation
Abstract
T-cell activation is the net product of competing positive and negative signals transduced by regulatory molecules on antigen-presenting cells (APCs) binding to corresponding ligands on T cells. Having previously identified DC-HIL as a receptor expressed by APCs that contains an extracellular immunoglobulin (Ig)-like domain, we postulated that it plays a role in T-cell activation. To probe this function, we created soluble recombinant DC-HIL, which we observed to bind activated (but not resting) T cells, indicating that expression of the putative ligand on T cells is induced by activation. Binding of DC-HIL to naive T cells attenuated these cells' primary response to anti-CD3 antibody, curtailing IL-2 production, and preventing entry into the cell cycle. DC-HIL also inhibited reactivation of T cells previously activated by APCs (secondary response). By contrast, addition of soluble DC-HIL to either allogeneic or ovalbumin-specific lymphocyte reactions augmented T-cell proliferation, and its injection into mice during the elicitation (but not sensitization) phase of contact hypersensitivity exacerbated ear-swelling responses. Mutant analyses showed the inhibitory function of DC-HIL to reside in its extracellular Ig-like domain. We conclude that endogenous DC-HIL is a negative regulator of T lymphocyte activation, and that this native inhibitory function can be blocked by exogenous DC-HIL, leading to enhanced immune responses.
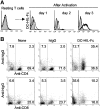
Figures






Similar articles
-
Syndecan-4 mediates the coinhibitory function of DC-HIL on T cell activation.J Immunol. 2007 Nov 1;179(9):5778-84. doi: 10.4049/jimmunol.179.9.5778. J Immunol. 2007. PMID: 17947650
-
DC-HIL/glycoprotein Nmb promotes growth of melanoma in mice by inhibiting the activation of tumor-reactive T cells.Cancer Res. 2010 Jul 15;70(14):5778-87. doi: 10.1158/0008-5472.CAN-09-2538. Epub 2010 Jun 22. Cancer Res. 2010. PMID: 20570888 Free PMC article.
-
The DC-HIL/syndecan-4 pathway inhibits human allogeneic T-cell responses.Eur J Immunol. 2009 Apr;39(4):965-74. doi: 10.1002/eji.200838990. Eur J Immunol. 2009. PMID: 19350579 Free PMC article.
-
Binding of DC-HIL to dermatophytic fungi induces tyrosine phosphorylation and potentiates antigen presenting cell function.J Immunol. 2009 Oct 15;183(8):5190-8. doi: 10.4049/jimmunol.0901319. Epub 2009 Sep 30. J Immunol. 2009. PMID: 19794069
-
Depleting syndecan-4+ T lymphocytes using toxin-bearing dendritic cell-associated heparan sulfate proteoglycan-dependent integrin ligand: a new opportunity for treating activated T cell-driven disease.J Immunol. 2010 Apr 1;184(7):3554-61. doi: 10.4049/jimmunol.0903250. Epub 2010 Feb 22. J Immunol. 2010. PMID: 20176742 Free PMC article.
Cited by
-
Investigating the role and regulation of GPNMB in progranulin-deficient macrophages.Front Immunol. 2024 Sep 26;15:1417836. doi: 10.3389/fimmu.2024.1417836. eCollection 2024. Front Immunol. 2024. PMID: 39391322 Free PMC article.
-
The soluble glycoprotein NMB (GPNMB) produced by macrophages induces cancer stemness and metastasis via CD44 and IL-33.Cell Mol Immunol. 2021 Mar;18(3):711-722. doi: 10.1038/s41423-020-0501-0. Epub 2020 Jul 29. Cell Mol Immunol. 2021. PMID: 32728200 Free PMC article.
-
The transcription factor MITF is a critical regulator of GPNMB expression in dendritic cells.Cell Commun Signal. 2015 Mar 24;13:19. doi: 10.1186/s12964-015-0099-5. Cell Commun Signal. 2015. PMID: 25889792 Free PMC article.
-
The immune inhibitory receptor osteoactivin is upregulated in monocyte-derived dendritic cells by BCR-ABL tyrosine kinase inhibitors.Cancer Immunol Immunother. 2012 Feb;61(2):193-202. doi: 10.1007/s00262-011-1096-1. Epub 2011 Aug 27. Cancer Immunol Immunother. 2012. PMID: 21874302 Free PMC article.
-
Glycoprotein Nonmetastatic Melanoma Protein B (GPNMB) Ameliorates the Inflammatory Response in Periodontal Disease.Inflammation. 2019 Aug;42(4):1170-1178. doi: 10.1007/s10753-019-00977-4. Inflammation. 2019. PMID: 30793225
References
-
- Chambers CA, Allison JP. Co-stimulation in T cell responses. Curr Opin Immunol. 1997;9:396–404. - PubMed
-
- Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3:939–951. - PubMed
-
- Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. - PubMed
-
- Tivol EA, Borriello F, Schweitzer AN, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

