BMP4, SCF, and hypoxia cooperatively regulate the expansion of murine stress erythroid progenitors
- PMID: 17284534
- PMCID: PMC1885504
- DOI: 10.1182/blood-2006-04-016154
BMP4, SCF, and hypoxia cooperatively regulate the expansion of murine stress erythroid progenitors
Abstract
The erythroid response to acute anemia relies on the rapid expansion in the spleen of a specialized population of erythroid progenitors termed stress BFU-E. This expansion requires BMP4/Madh5-dependent signaling in vivo; however, in vitro, BMP4 alone cannot recapitulate the expansion of stress BFU-E observed in vivo, which suggests that other signals are required. In this report we show that mutation of the Kit receptor results in a severe defect in the expansion of stress BFU-E, indicating a role for the Kit/SCF signaling pathway in stress erythropoiesis. In vitro analysis showed that BMP4 and SCF are necessary for the expansion of stress BFU-E, but only when spleen cells were cultured in BMP4 + SCF at low-oxygen concentrations did we recapitulate the expansion of stress BFU-E observed in vivo. Culturing spleen cells in BMP4, SCF under hypoxic conditions resulted in the preferential expansion of erythroid progenitors characterized by the expression of Kit, CD71, and TER119. This expression pattern is also seen in stress erythroid progenitors isolated from patients with sickle cell anemia and patients with beta-thalassemia. Taken together these data demonstrate that SCF and hypoxia synergize with BMP4 to promote the expansion and differentiation of stress BFU-E during the recovery from acute anemia.
Figures
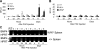
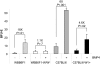


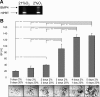


References
-
- Lenox L, Perry J, Paulson R. BMP4 and Madh5 regulate the erythroid response to acute anemia. Blood. 2005;105:2741–2748. - PubMed
-
- Bernstein A, Forrester L, Reith AD, Dubreuil P, Rottapel R. The murine W/c-kit and Steel loci and the control of hematopoiesis. Semin Hematol. 1991;28:138–142. - PubMed
-
- Russell ES. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. - PubMed
-
- Ashman LK. The biology of stem cell factor and its receptor C-kit. Int J Biochem Cell Biol. 1999;31:1037–1051. - PubMed
-
- Huang E, Nocka K, Beier DR, et al. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990;63:225–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

