Effects of a nucleoside reverse transcriptase inhibitor, stavudine, on glucose disposal and mitochondrial function in muscle of healthy adults
- PMID: 17284576
- PMCID: PMC3206591
- DOI: 10.1152/ajpendo.00550.2006
Effects of a nucleoside reverse transcriptase inhibitor, stavudine, on glucose disposal and mitochondrial function in muscle of healthy adults
Abstract
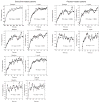
Mitochondrial dysfunction may contribute to the development of insulin resistance and type 2 diabetes. Nucleoside reverse transcriptase inhibitors (NRTIs), specifically stavudine, are known to alter mitochondrial function in human immunodeficiency virus (HIV)-infected individuals, but the effects of stavudine on glucose disposal and mitochondrial function in muscle have not been prospectively evaluated. In this study, we investigated short-term stavudine administration among healthy control subjects to determine effects on insulin sensitivity. A secondary aim was to determine the effects of stavudine on mitochondrial DNA (mtDNA) and function. Sixteen participants without personal or family history of diabetes were enrolled. Subjects were randomized to receive stavudine, 30-40 mg, twice a day, or placebo for 1 mo. Insulin sensitivity determined by glucose infusion rate during the hyperinsulinemic euglycemic clamp was significantly reduced after 1-mo exposure in the stavudine-treated subjects compared with placebo (-0.8 +/- 0.5 vs. +0.7 +/- 0.3 mg.kg(-1).min(-1), P = 0.04, stavudine vs. placebo). In addition, muscle biopsy specimens in the stavudine-treated group showed significant reduction in mtDNA/nuclear DNA (-52%, P = 0.005), with no change in placebo-treated subjects (+8%, P = 0.9). (31)P magnetic resonance spectroscopy (MRS) studies of mitochondrial function correlated with insulin sensitivity measures (r2 = 0.5, P = 0.008). These findings demonstrate that stavudine administration has potent effects on insulin sensitivity among healthy subjects. Further studies are necessary to determine whether changes in mtDNA resulting from stavudine contribute to effects on insulin sensitivity.
Figures




References
-
- Côté HC, Brumme ZL, Craib KJ, Alexander CS, Wynhoven B, Ting L, Wong H, Harris M, Harrigan PR, O’Shaughnessy MV, Montaner JS. Changes in mitochondrial DNA as a marker of nucleoside toxicity in HIV-infected patients. N Engl J Med. 2002;346:811–820. - PubMed
-
- Gan SK, Samaras K, Thompson CH, Kraegen EW, Carr A, Cooper DA, Chisholm DJ. Altered myocellular and abdominal fat partitioning predict disturbance in insulin action in HIV protease inhibitor-related lipodystrophy. Diabetes. 2002;51:3163–3169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

