Eukaryotic RNase P RNA mediates cleavage in the absence of protein
- PMID: 17284611
- PMCID: PMC1892975
- DOI: 10.1073/pnas.0607326104
Eukaryotic RNase P RNA mediates cleavage in the absence of protein
Erratum in
- Proc Natl Acad Sci U S A. 2009 May 12;106(19):8078
Abstract
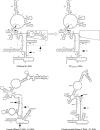
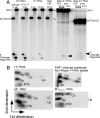
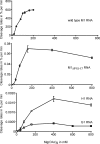
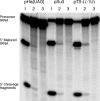
The universally conserved ribonucleoprotein RNase P is involved in the processing of tRNA precursor transcripts. RNase P consists of one RNA and, depending on its origin, a variable number of protein subunits. Catalytic activity of the RNA moiety so far has been demonstrated only for bacterial and some archaeal RNase P RNAs but not for their eukaryotic counterparts. Here, we show that RNase P RNAs from humans and the lower eukaryote Giardia lamblia mediate cleavage of four tRNA precursors and a model RNA hairpin loop substrate in the absence of protein. Compared with bacterial RNase P RNA, the rate of cleavage (k(obs)) was five to six orders of magnitude lower, whereas the affinity for the substrate (appK(d)) was reduced approximately 20- to 50-fold. We conclude that the RNA-based catalytic activity of RNase P has been preserved during evolution. This finding opens previously undescribed ways to study the role of the different proteins subunits of eukaryotic RNase P.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
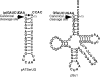
Comment in
-
Uniformity amid diversity in RNase P.Proc Natl Acad Sci U S A. 2007 Feb 13;104(7):2031-2. doi: 10.1073/pnas.0611193104. Epub 2007 Feb 7. Proc Natl Acad Sci U S A. 2007. PMID: 17287341 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

