Identification of novel peptide hormones in the human proteome by hidden Markov model screening
- PMID: 17284679
- PMCID: PMC1800923
- DOI: 10.1101/gr.5755407
Identification of novel peptide hormones in the human proteome by hidden Markov model screening
Abstract
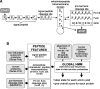
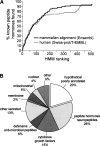
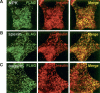
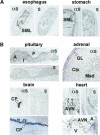
Peptide hormones are small, processed, and secreted peptides that signal via membrane receptors and play critical roles in normal and pathological physiology. The search for novel peptide hormones has been hampered by their small size, low or restricted expression, and lack of sequence similarity. To overcome these difficulties, we developed a bioinformatics search tool based on the hidden Markov model formalism that uses several peptide hormone sequence features to estimate the likelihood that a protein contains a processed and secreted peptide of this class. Application of this tool to an alignment of mammalian proteomes ranked 90% of known peptide hormones among the top 300 proteins. An analysis of the top scoring hypothetical and poorly annotated human proteins identified two novel candidate peptide hormones. Biochemical analysis of the two candidates, which we called spexin and augurin, showed that both were localized to secretory granules in a transfected pancreatic cell line and were recovered from the cell supernatant. Spexin was expressed in the submucosal layer of the mouse esophagus and stomach, and a predicted peptide from the spexin precursor induced muscle contraction in a rat stomach explant assay. Augurin was specifically expressed in mouse endocrine tissues, including pituitary and adrenal gland, choroid plexus, and the atrio-ventricular node of the heart. Our findings demonstrate the utility of a bioinformatics approach to identify novel biologically active peptides. Peptide hormones and their receptors are important diagnostic and therapeutic targets, and our results suggest that spexin and augurin are novel peptide hormones likely to be involved in physiological homeostasis.
Figures


Similar articles
-
Evolutionary sequence modeling for discovery of peptide hormones.PLoS Comput Biol. 2009 Jan;5(1):e1000258. doi: 10.1371/journal.pcbi.1000258. Epub 2009 Jan 9. PLoS Comput Biol. 2009. PMID: 19132080 Free PMC article.
-
Expression of the spexin gene in the rat adrenal gland and evidences suggesting that spexin inhibits adrenocortical cell proliferation.Peptides. 2010 Apr;31(4):676-82. doi: 10.1016/j.peptides.2009.12.025. Epub 2010 Jan 4. Peptides. 2010. PMID: 20045034
-
Evaluation of signal peptide prediction algorithms for identification of mycobacterial signal peptides using sequence data from proteomic methods.Microbiology (Reading). 2009 Jul;155(Pt 7):2375-2383. doi: 10.1099/mic.0.025270-0. Epub 2009 Apr 23. Microbiology (Reading). 2009. PMID: 19389770 Free PMC article.
-
Complex and pleiotropic signaling pathways regulated by the secreted protein augurin.Cell Commun Signal. 2023 Apr 11;21(1):69. doi: 10.1186/s12964-023-01090-8. Cell Commun Signal. 2023. PMID: 37041625 Free PMC article. Review.
-
Ghrelin expression and actions: a novel peptide for an old cell type of the diffuse endocrine system.Exp Biol Med (Maywood). 2004 Nov;229(10):1007-16. doi: 10.1177/153537020422901004. Exp Biol Med (Maywood). 2004. PMID: 15522836 Review.
Cited by
-
Overexpression of ECRG4 enhances chemosensitivity to 5-fluorouracil in the human gastric cancer SGC-7901 cell line.Tumour Biol. 2013 Aug;34(4):2269-73. doi: 10.1007/s13277-013-0768-1. Epub 2013 Apr 4. Tumour Biol. 2013. PMID: 23553029
-
Nonalcoholic Fatty Liver Disease: Lipids and Insulin Resistance.Clin Liver Dis. 2016 May;20(2):245-62. doi: 10.1016/j.cld.2015.10.007. Epub 2016 Feb 18. Clin Liver Dis. 2016. PMID: 27063267 Free PMC article. Review.
-
Modulatory effect of olanzapine on SMIM20/phoenixin, NPQ/spexin and NUCB2/nesfatin-1 gene expressions in the rat brainstem.Pharmacol Rep. 2021 Aug;73(4):1188-1194. doi: 10.1007/s43440-021-00267-7. Epub 2021 Apr 29. Pharmacol Rep. 2021. PMID: 33928538 Free PMC article.
-
CSF Proteomics of Patients with Hydrocephalus and Subarachnoid Haemorrhage.Transl Neurosci. 2019 Oct 2;10:244-253. doi: 10.1515/tnsci-2019-0040. eCollection 2019. Transl Neurosci. 2019. PMID: 31637049 Free PMC article.
-
Deorphanization of novel peptides and their receptors.AAPS J. 2010 Sep;12(3):378-84. doi: 10.1208/s12248-010-9198-9. Epub 2010 May 6. AAPS J. 2010. PMID: 20446073 Free PMC article. Review.
References
-
- Baggerman G., Liu F., Wets G., Schoofs L., Liu F., Wets G., Schoofs L., Wets G., Schoofs L., Schoofs L. Bioinformatic analysis of peptide precursor proteins. Ann. N. Y. Acad. Sci. 2005;1040:59–65. - PubMed
-
- Braun-Menendez E., Fasciolo J.C., Leloir L.F., Muñoz J.M., Fasciolo J.C., Leloir L.F., Muñoz J.M., Leloir L.F., Muñoz J.M., Muñoz J.M. Hypertensin: The substance causing renal hypertension. Nature. 1939;144:980–981.
-
- Burge C., Karlin S., Karlin S. Prediction of complete gene structures in human genomic DNA. J. Mol. Biol. 1997;268:78–94. - PubMed
-
- Burgus R., Dunn T.F., Desiderio D., Guillemin R., Dunn T.F., Desiderio D., Guillemin R., Desiderio D., Guillemin R., Guillemin R. Molecular structure of the hypothalamic hypophysiotropic TRF factor of ovine origin: Mass spectrometry demonstration of the PCA-His-Pro-NH2 sequence. C. R. Hebd. Seances Acad. Sci. 1969;269:1870–1873. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
