Extracellular proteolysis in transgenic mouse models of breast cancer
- PMID: 17286208
- PMCID: PMC1820839
- DOI: 10.1007/s10911-007-9040-x
Extracellular proteolysis in transgenic mouse models of breast cancer
Abstract
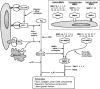
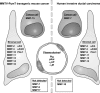
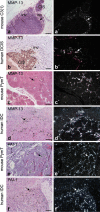
Growth and invasion of breast cancer require extracellular proteolysis in order to physically restructure the tissue microenvironment of the mammary gland. This pathological tissue remodeling process depends on a collaboration of epithelial and stromal cells. In fact, the majority of extracellular proteases are provided by stromal cells rather than cancer cells. This distinct expression pattern is seen in human breast cancers and also in transgenic mouse models of breast cancer. The similar expression patterns suggest that transgenic mouse models are ideally suited to study the role of extracellular proteases in cancer progression. Here we give a status report on protease intervention studies in transgenic models. These studies demonstrate that proteases are involved in all stages of breast cancer progression from carcinogenesis to metastasis. Transgenic models are now beginning to provide vital mechanistic insight that will allow us to combat breast cancer invasion and metastasis with new protease-targeted drugs.
Figures
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1002/bies.20281', 'is_inner': False, 'url': 'https://doi.org/10.1002/bies.20281'}, {'type': 'PubMed', 'value': '16108064', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16108064/'}]}
- Green KA, Lund LR. ECM degrading proteases and tissue remodelling in the mammary gland. BioEssays 2005;27:894–903. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0955-0674(98)80044-6', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0955-0674(98)80044-6'}, {'type': 'PubMed', 'value': '9818179', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9818179/'}]}
- Johnsen M, Lund LR, Rømer J, Almholt K, Danø K. Cancer invasion and tissue remodeling: common themes in proteolytic matrix degradation. Curr Opin Cell Biol 1998;10:667–71. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1007/s000180050497', 'is_inner': False, 'url': 'https://doi.org/10.1007/s000180050497'}, {'type': 'PMC', 'value': 'PMC11146824', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC11146824/'}, {'type': 'PubMed', 'value': '10949579', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10949579/'}]}
- Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci 2000;57:25–40. - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1042/BST0300189', 'is_inner': False, 'url': 'https://doi.org/10.1042/bst0300189'}, {'type': 'PubMed', 'value': '12023849', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12023849/'}]}
- Bass R, Ellis V. Cellular mechanisms regulating non-haemostatic plasmin generation. Biochem Soc Trans 2002;30:189–94. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '11003846', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11003846/'}]}
- Lund LR, Bjørn SF, Sternlicht MD, Nielsen BS, Solberg H, Usher PA, et al. Lactational competence and involution of the mouse mammary gland require plasminogen. Development 2000;127:4481–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

