CYP2C8 and antimalaria drug efficacy
- PMID: 17286541
- PMCID: PMC7117598
- DOI: 10.2217/14622416.8.2.187
CYP2C8 and antimalaria drug efficacy
Abstract
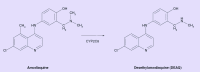
Malaria is a major infectious disease. In the last 10 years it has killed more than 20 million people, mainly small children in Africa. The highly efficacious artemisinine combination therapy is being launched globally, constituting the main hope for fighting the disease. Amodiaquine is a main partner in these combinations. Amodiaquine is almost entirely metabolized by the polymorphic cytochrome P450 (CYP) isoform 2C8 to the pharmacologically active desethylamodiaquine. The question remains whether the efficacy of amodiaquine is affected by the gene polymorphism. Genotype-inferred low metabolizers are found in 1-4% of African populations, which corresponds to millions of expected exposures to the drug. In vivo pharmacokinetic data on amodiaquine is limited. By combining it with published in vitro pharmacodynamic and drug metabolism information, we review and predict the possible relevance, or lack of, of CYP2C8 polymorphisms in the present and future efficacy of amodiaquine. Chloroquine and dapsone, both substrates of CYP2C8, are also discussed in the same context.
Figures

References
Bibliography
-
- Breman JG. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am. J. Trop. Med. Hyg. 64(Suppl. 1–2) 1–11 (2001). - PubMed
-
- Sachs J, Malaney P: The economic and social burden of malaria. Nature 415(6872) 680–685 (2002) - PubMed
-
Valuable review on the global burden of malaria.
-
- Girard MP, Reed ZH, Friede M, Kieny MP: A review of human vaccine research and development: malaria. Vaccine (2006) (Epub ahead of print). - PubMed
-
- Nosten F, Brasseur P: Combination therapy for malaria: the way forward? Drugs 62(9) 1315–1329 (2002). - PubMed
-
Excellent review on the concept of artemisinine combined therapy.
-
- Li XQ, Bjorkman A, Andersson TB, Ridderstrom M, Masimirembwa CM: Amodiaquine clearance and its metabolism to N-desethylamodiaquine is mediated by CYP2C8: a new high affinity and turnover enzyme-specific probe substrate. J. Pharmacol. Exp. Ther. 300(2) 399–407 (2002). - PubMed
-
Initial and very complete study pointing to cytochrome P450 (CYP)2C8 as the main enzyme in the metabolism of amodiaquine (AQ).
Websites
-
- Human Cytochrome P450 Allele Nomenclature Committee. www.cypalleles.ki.se/
-
- WHO: Global Malaria Program – treatment policies. www.who.int/malaria/treatmentpolicies.html
-
Up-to-date database of the national malaria treatment policies.
-
- Medicines for Malaria Venture. www.mmv.org
-
- US Institute of Health artesunate and amodiaquine fixed-dose combination in the treatment of uncomplicated Plasmodium falciparum malaria study. www.clinicaltrials.gov/ct/show/NCT00316 329
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
