Affinity maturation of immunoglobulin A anti-tissue transglutaminase autoantibodies during development of coeliac disease
- PMID: 17286799
- PMCID: PMC1868886
- DOI: 10.1111/j.1365-2249.2007.03336.x
Affinity maturation of immunoglobulin A anti-tissue transglutaminase autoantibodies during development of coeliac disease
Abstract
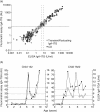
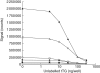
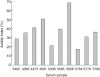
Coeliac disease (CD) is an immune-mediated enteropathy triggered by ingestion of wheat gluten and related cereals in genetically predisposed individuals. Circulating immunoglobulin A (IgA) class autoantibodies against tissue transglutaminase (IgA-TGA) are highly specific and sensitive serological markers for CD, which is ultimately confirmed by duodenal biopsy. Although CD is considered a life-long disorder, transient or fluctuating IgA-TGA seropositivity has been observed in asymptomatic individuals on a gluten-containing diet. We set out to explore possible differences in the maturation of IgA-TGA avidity between individuals progressing to CD and subjects remaining healthy despite occasional expression of autoantibodies. We developed a time-resolved fluorometric IgA-TGA assay based on human recombinant tissue transglutaminase (tTG), and further modified the method to also measure urea-dependent avidity of the autoantibodies. We measured the autoantibody titres and avidities of sequential serum samples from 10 children developing CD and 10 children presenting transient or fluctuating autoantibodies. Both the initial titres at seroconversion and peak values of transient/fluctuating IgA-TGA were significantly lower than corresponding autoantibody titres in samples drawn from individuals with progressing CD (P = 0.004 and P = 0.0002, respectively). However, there were no statistically significant differences in the initial or peak avidity index values between the two groups of children. The avidity index values increased during the follow-up period (P = 0.013 for both groups) with no significant difference in the rate of avidity maturation between children with transient/fluctuating IgA-TGA and children developing CD. According to our results, high autoantibody titres have a higher predictive value than avidity maturation of TGA-IgA in screening for CD.
Figures



Similar articles
-
Elevation of IgG antibodies against tissue transglutaminase as a diagnostic tool for coeliac disease in selective IgA deficiency.Gut. 2003 Nov;52(11):1567-71. doi: 10.1136/gut.52.11.1567. Gut. 2003. PMID: 14570724 Free PMC article.
-
Prediction of clinical and mucosal severity of coeliac disease and dermatitis herpetiformis by quantification of IgA/IgG serum antibodies to tissue transglutaminase.J Pediatr Gastroenterol Nutr. 2010 Feb;50(2):140-6. doi: 10.1097/MPG.0b013e3181a81384. J Pediatr Gastroenterol Nutr. 2010. PMID: 19841593
-
Antibodies against human tissue transglutaminase and endomysium in diagnosing and monitoring coeliac disease.Scand J Gastroenterol. 2002 Jun;37(6):685-91. doi: 10.1080/00365520212496. Scand J Gastroenterol. 2002. PMID: 12126247
-
IgA anti-tissue transglutaminase: setting the stage for coeliac disease screening.Eur J Gastroenterol Hepatol. 2001 Jun;13(6):635-7. doi: 10.1097/00042737-200106000-00004. Eur J Gastroenterol Hepatol. 2001. PMID: 11434587 Review.
-
Tissue transglutaminase in celiac disease: role of autoantibodies.Amino Acids. 2009 Apr;36(4):693-9. doi: 10.1007/s00726-008-0120-z. Epub 2008 Jul 4. Amino Acids. 2009. PMID: 18600381 Review.
Cited by
-
Anti-Lipid IgG Antibodies Are Produced via Germinal Centers in a Murine Model Resembling Human Lupus.Front Immunol. 2016 Sep 29;7:396. doi: 10.3389/fimmu.2016.00396. eCollection 2016. Front Immunol. 2016. PMID: 27746783 Free PMC article.
-
Microarray-Based Avidity Assay for Assessment of Thyroid Autoantibodies.Diagnostics (Basel). 2025 Jan 31;15(3):341. doi: 10.3390/diagnostics15030341. Diagnostics (Basel). 2025. PMID: 39941271 Free PMC article.
-
Anti-transglutaminase antibodies in non-coeliac children suffering from infectious diseases.Clin Exp Immunol. 2010 Feb;159(2):217-23. doi: 10.1111/j.1365-2249.2009.04054.x. Epub 2009 Nov 12. Clin Exp Immunol. 2010. PMID: 19912255 Free PMC article.
-
Serum autoantibodies directed against transglutaminase-2 have a low avidity compared with alloantibodies against gliadin in coeliac disease.Clin Exp Immunol. 2014 Jul;177(1):86-93. doi: 10.1111/cei.12302. Clin Exp Immunol. 2014. PMID: 24666357 Free PMC article.
References
-
- Dewar D, Pereira SP, Ciclitira PJ. The pathogenesis of coeliac disease. Int J Biochem Cell Biol. 2004;36:17–24. - PubMed
-
- Brandimarte G, Tursi A, Giorgetti GM. Changing trends in clinical form of celiac disease. Which is now the main form of celiac disease in clinical practice? Minerva Gastroenterol Dietol. 2002;48:121–30. - PubMed
-
- Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002;346:180–8. - PubMed
-
- Csizmadia CG, Mearin ML, von Blomberg BM, Brand R, Verloove-Vanhorick SP. An iceberg of childhood coeliac disease in the Netherlands. Lancet. 1999;353:813–4. - PubMed
-
- Hill I, Fasano A, Schwartz R, Counts D, Glock M, Horvath K. The prevalence of celiac disease in at-risk groups of children in the United States. J Pediatr. 2000;136:86–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

