The Snail repressor is required for PMC ingression in the sea urchin embryo
- PMID: 17287249
- PMCID: PMC3045531
- DOI: 10.1242/dev.02805
The Snail repressor is required for PMC ingression in the sea urchin embryo
Abstract
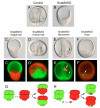
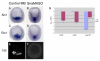
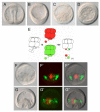
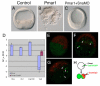
In metazoans, the epithelial-mesenchymal transition (EMT) is a crucial process for placing the mesoderm beneath the ectoderm. Primary mesenchyme cells (PMCs) at the vegetal pole of the sea urchin embryo ingress into the floor of the blastocoele from the blastula epithelium and later become the skeletogenic mesenchyme. This ingression movement is a classic EMT during which the PMCs penetrate the basal lamina, lose adherens junctions and migrate into the blastocoele. Later, secondary mesenchyme cells (SMCs) also enter the blastocoele via an EMT, but they accompany the invagination of the archenteron initially, in much the same way vertebrate mesenchyme enters the embryo along with endoderm. Here we identify a sea urchin ortholog of the Snail transcription factor, and focus on its roles regulating EMT during PMC ingression. Functional knockdown analyses of Snail in whole embryos and chimeras demonstrate that Snail is required in micromeres for PMC ingression. Snail represses the transcription of cadherin, a repression that appears evolutionarily conserved throughout the animal kingdom. Furthermore, Snail expression is required for endocytosis of cadherin, a cellular activity that accompanies PMC ingression. Perturbation studies position Snail in the sea urchin micromere-PMC gene regulatory network (GRN), downstream of Pmar1 and Alx1, and upstream of several PMC-expressed proteins. Taken together, our findings indicate that Snail plays an essential role in PMCs to control the EMT process, in part through its repression of cadherin expression during PMC ingression, and in part through its role in the endocytosis that helps convert an epithelial cell to a mesenchyme cell.
Figures




Similar articles
-
Ingression of primary mesenchyme cells of the sea urchin embryo: a precisely timed epithelial mesenchymal transition.Birth Defects Res C Embryo Today. 2007 Dec;81(4):241-52. doi: 10.1002/bdrc.20113. Birth Defects Res C Embryo Today. 2007. PMID: 18228256 Review.
-
A homologue of snail is expressed transiently in subsets of mesenchyme cells in the sea urchin embryo and is down-regulated in axis-deficient embryos.Dev Dyn. 2006 Nov;235(11):3121-31. doi: 10.1002/dvdy.20941. Dev Dyn. 2006. PMID: 16958110
-
The control of foxN2/3 expression in sea urchin embryos and its function in the skeletogenic gene regulatory network.Development. 2011 Mar;138(5):937-45. doi: 10.1242/dev.058396. Development. 2011. PMID: 21303847 Free PMC article.
-
Primary mesenchyme cell patterning during the early stages following ingression.Dev Biol. 2003 Feb 1;254(1):68-78. doi: 10.1016/s0012-1606(02)00025-8. Dev Biol. 2003. PMID: 12606282
-
Mechanisms of the epithelial-to-mesenchymal transition in sea urchin embryos.Tissue Barriers. 2015 Jun 17;3(4):e1059004. doi: 10.1080/21688370.2015.1059004. eCollection 2015 Oct-Dec. Tissue Barriers. 2015. PMID: 26716069 Free PMC article. Review.
Cited by
-
Characterization and Endocytic Internalization of Epith-2 Cell Surface Glycoprotein during the Epithelial-to-Mesenchymal Transition in Sea Urchin Embryos.Front Endocrinol (Lausanne). 2013 Aug 30;4:112. doi: 10.3389/fendo.2013.00112. eCollection 2013. Front Endocrinol (Lausanne). 2013. PMID: 24009602 Free PMC article.
-
Role of snail activation in alcohol-induced iNOS-mediated disruption of intestinal epithelial cell permeability.Alcohol Clin Exp Res. 2011 Sep;35(9):1635-43. doi: 10.1111/j.1530-0277.2011.01510.x. Epub 2011 Apr 27. Alcohol Clin Exp Res. 2011. PMID: 21535025 Free PMC article.
-
Morphogenesis in sea urchin embryos: linking cellular events to gene regulatory network states.Wiley Interdiscip Rev Dev Biol. 2012 Mar-Apr;1(2):231-52. doi: 10.1002/wdev.18. Epub 2011 Dec 27. Wiley Interdiscip Rev Dev Biol. 2012. PMID: 23801438 Free PMC article. Review.
-
From genome to anatomy: The architecture and evolution of the skeletogenic gene regulatory network of sea urchins and other echinoderms.Genesis. 2018 Oct;56(10):e23253. doi: 10.1002/dvg.23253. Genesis. 2018. PMID: 30264451 Free PMC article. Review.
-
Sub-circuits of a gene regulatory network control a developmental epithelial-mesenchymal transition.Development. 2014 Apr;141(7):1503-13. doi: 10.1242/dev.101436. Epub 2014 Mar 5. Development. 2014. PMID: 24598159 Free PMC article.
References
-
- Alberga A, Boulay JL, Kempe E, Dennefeld C, Haenlin M. The snail gene required for mesoderm formation in Drosophila is expressed dynamically in derivatives of all three germ layers. Development. 1991;111:983–992. - PubMed
-
- Amore G, Yavrouian RG, Peterson KJ, Ransick A, McClay DR, Davidson EH. Spdeadringer, a sea urchin embryo gene required separately in skeletogenic and oral ectoderm gene regulatory networks. Dev. Biol. 2003;261:55–81. - PubMed
-
- Angerer LM, Angerer RC. Patterning the sea urchin embryo: gene regulatory networks, signaling pathways, and cellular interactions. Curr. Top. Dev. Biol. 2003;53:159–198. - PubMed
-
- Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. - PubMed
-
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of Ecadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2000;2:84–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

