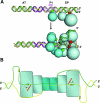
Model for T-antigen-dependent melting of the simian virus 40 core origin based on studies of the interaction of the beta-hairpin with DNA
- PMID: 17287270
- PMCID: PMC1900137
- DOI: 10.1128/JVI.02451-06
Model for T-antigen-dependent melting of the simian virus 40 core origin based on studies of the interaction of the beta-hairpin with DNA
Abstract
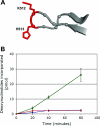
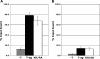
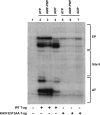
The interaction of simian virus 40 (SV40) T antigen (T-ag) with the viral origin has served as a model for studies of site-specific recognition of a eukaryotic replication origin and the mechanism of DNA unwinding. These studies have revealed that a motif termed the "beta-hairpin" is necessary for assembly of T-ag on the SV40 origin. Herein it is demonstrated that residues at the tip of the "beta-hairpin" are needed to melt the origin-flanking regions and that the T-ag helicase domain selectively assembles around one of the newly generated single strands in a manner that accounts for its 3'-to-5' helicase activity. Furthermore, T-ags mutated at the tip of the "beta-hairpin" are defective for oligomerization on duplex DNA; however, they can assemble on hybrid duplex DNA or single-stranded DNA (ssDNA) substrates provided the strand containing the 3' extension is present. Collectively, these experiments indicate that residues at the tip of the beta-hairpin generate ssDNA in the core origin and that the ssDNA is essential for subsequent oligomerization events.
Figures



References
-
- Ahnert, P., and S. S. Patel. 1997. Asymmetric interactions of hexameric bacteriophage T7 DNA helicase with the 5′- and 3′-tails of the forked DNA substrate. J. Biol. Chem. 272:32267-32273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

