The nucleocytoplasmic rabies virus P protein counteracts interferon signaling by inhibiting both nuclear accumulation and DNA binding of STAT1
- PMID: 17287281
- PMCID: PMC1866157
- DOI: 10.1128/JVI.01930-06
The nucleocytoplasmic rabies virus P protein counteracts interferon signaling by inhibiting both nuclear accumulation and DNA binding of STAT1
Abstract
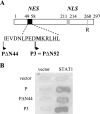
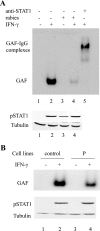
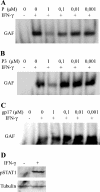
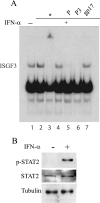
Rabies virus P protein inhibits alpha interferon (IFN-alpha)- and IFN-gamma-stimulated Jak-STAT signaling by retaining phosphorylated STAT1 in the cytoplasm. Here, we show that P also blocks an intranuclear step that is the STAT1 binding to the DNA promoter of IFN-responsive genes. As P is a nucleocytoplasmic shuttling protein, we first investigated the effect of the cellular distribution of P on the localization of STAT1 and consequently on IFN signaling. We show that the localization of STAT1 is correlated with the localization of P: in cells expressing a nuclear form of P (the short P3 isoform or the complete P in the presence of the export inhibitor leptomycin B), STAT1 is nuclear, whereas in cells expressing a cytoplasmic form of P, STAT1 is cytoplasmic. However, the expression of nuclear forms of P inhibits the signaling of both IFN-gamma and IFN-alpha, demonstrating that the retention of STAT1 in the cytoplasm is not the only mechanism involved in the inhibition of IFN signaling. Electrophoretic mobility shift analysis indicates that P expression in the cell extracts of infected cells or in stable cell lines prevents IFN-induced DNA binding of STAT1. The loss of the DNA binding of STAT1 and ISGF3 was also observed when purified recombinant P or P3 was added to the extracts of IFN-gamma- or IFN-alpha-treated cells, indicating that P directly affects the DNA binding activity of STAT1. Then products of the rabies virus P gene are able to counteract IFN signaling by creating both cytoplasmic and nuclear blocks for STAT1.
Figures
Similar articles
-
Rabies virus P protein interacts with STAT1 and inhibits interferon signal transduction pathways.J Virol. 2005 Nov;79(22):14411-20. doi: 10.1128/JVI.79.22.14411-14420.2005. J Virol. 2005. PMID: 16254375 Free PMC article.
-
IFN-type-I-mediated signaling is regulated by modulation of STAT2 nuclear export.J Cell Sci. 2006 Mar 15;119(Pt 6):1092-104. doi: 10.1242/jcs.02822. Epub 2006 Feb 28. J Cell Sci. 2006. PMID: 16507591
-
Nipah virus V and W proteins have a common STAT1-binding domain yet inhibit STAT1 activation from the cytoplasmic and nuclear compartments, respectively.J Virol. 2004 Jun;78(11):5633-41. doi: 10.1128/JVI.78.11.5633-5641.2004. J Virol. 2004. PMID: 15140960 Free PMC article.
-
Rabies viral mechanisms to escape the IFN system: the viral protein P interferes with IRF-3, Stat1, and PML nuclear bodies.J Interferon Cytokine Res. 2006 May;26(5):271-80. doi: 10.1089/jir.2006.26.271. J Interferon Cytokine Res. 2006. PMID: 16689655 Review.
-
Stat1-mediated cytoplasmic attenuation in osteoimmunology.J Cell Biochem. 2005 Feb 1;94(2):232-40. doi: 10.1002/jcb.20316. J Cell Biochem. 2005. PMID: 15546140 Review.
Cited by
-
Lyssavirus P-protein selectively targets STAT3-STAT1 heterodimers to modulate cytokine signalling.PLoS Pathog. 2020 Sep 9;16(9):e1008767. doi: 10.1371/journal.ppat.1008767. eCollection 2020 Sep. PLoS Pathog. 2020. PMID: 32903273 Free PMC article.
-
IRF2 Cooperates with Phosphoprotein of Spring Viremia of Carp Virus to Suppress Antiviral Response in Zebrafish.J Virol. 2022 Nov 23;96(22):e0131422. doi: 10.1128/jvi.01314-22. Epub 2022 Oct 31. J Virol. 2022. PMID: 36314827 Free PMC article.
-
Genomic characterization, phylogenetic position and in situ localization of a novel putative mononegavirus in Lepeophtheirus salmonis.Arch Virol. 2019 Mar;164(3):675-689. doi: 10.1007/s00705-018-04119-3. Epub 2018 Dec 7. Arch Virol. 2019. PMID: 30535526 Free PMC article.
-
Intergenotypic replacement of lyssavirus matrix proteins demonstrates the role of lyssavirus M proteins in intracellular virus accumulation.J Virol. 2010 Feb;84(4):1816-27. doi: 10.1128/JVI.01665-09. Epub 2009 Dec 2. J Virol. 2010. PMID: 19955305 Free PMC article.
-
3Cpro of foot-and-mouth disease virus antagonizes the interferon signaling pathway by blocking STAT1/STAT2 nuclear translocation.J Virol. 2014 May;88(9):4908-20. doi: 10.1128/JVI.03668-13. Epub 2014 Feb 19. J Virol. 2014. PMID: 24554650 Free PMC article.
References
-
- Basler, C. F., and A. Garcia-Sastre. 2002. Viruses and the type I interferon antiviral system: induction and evasion. Int. Rev. Immunol. 21:305-337. - PubMed
-
- Blondel, D., T. Regad, N. Poisson, B. Pavie, H. Harper, P. P. Pandolfi, H. De Thé, and M. K. Chelbi-Alix. 2002. Rabies virus P and small P products interact directly with PML and reorganize PML nuclear bodies. Oncogene 21:7957-7970. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

