Eukaryotic ribosomal protein RPS25 interacts with the conserved loop region in a dicistroviral intergenic internal ribosome entry site
- PMID: 17287295
- PMCID: PMC1865070
- DOI: 10.1093/nar/gkl1121
Eukaryotic ribosomal protein RPS25 interacts with the conserved loop region in a dicistroviral intergenic internal ribosome entry site
Abstract
The intergenic region-internal ribosome entry site (IGR-IRES) of dicistroviruses binds to 40S ribosomal subunits in the absence of eukaryotic initiation factors (eIFs). Although the conserved loop sequences in dicistroviral IGR-IRES elements are protected from chemical modifications in the presence of the 40S subunit, molecular components in the 40S subunit, which interacts with the loop sequences in the IRES, have not been identified. Here, a chemical crosslinking study using 4-thiouridine-labeled IGR-IRES revealed interactions of the IGR-IRES with several 40S proteins but not with the 18S rRNA. The strongest crosslinking signal was identified for ribosomal protein S25 (rpS25). rpS25 is known to be a neighbor of rpS5, which has been shown to interact with a related IGR-IRES by cryo-electron microscopy. Crosslinking analysis with site-directed mutants showed that nucleotides UU(6089-6090), which are located in the loop region in conserved domain 2b in the IRES, appear to interact with rpS25. rpS25 is specific to eukaryotes, which explains why there is no recognition of the IGR-IRES by prokaryotic ribosomes. Although the idea that the IGR-IRES element may be a relict of a primitive translation system has been postulated, our experimental data suggest that the IRES has adapted to eukaryotic ribosomal proteins.
Figures


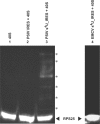
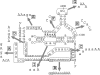

Similar articles
-
Factorless ribosome assembly on the internal ribosome entry site of cricket paralysis virus.J Mol Biol. 2002 Dec 13;324(5):889-902. doi: 10.1016/s0022-2836(02)01099-9. J Mol Biol. 2002. PMID: 12470947
-
Conserved element of the dicistrovirus IGR IRES that mimics an E-site tRNA/ribosome interaction mediates multiple functions.J Mol Biol. 2009 Mar 20;387(1):42-58. doi: 10.1016/j.jmb.2009.01.042. Epub 2009 Jan 29. J Mol Biol. 2009. PMID: 19361441
-
Ribosomal proteins mediate the hepatitis C virus IRES-HeLa 40S interaction.RNA. 2002 Jul;8(7):913-23. doi: 10.1017/s1355838202022057. RNA. 2002. PMID: 12166646 Free PMC article.
-
Translation initiation by factor-independent binding of eukaryotic ribosomes to internal ribosomal entry sites.C R Biol. 2005 Jul;328(7):589-605. doi: 10.1016/j.crvi.2005.02.004. C R Biol. 2005. PMID: 15992743 Review.
-
Comparing the three-dimensional structures of Dicistroviridae IGR IRES RNAs with other viral RNA structures.Virus Res. 2009 Feb;139(2):148-56. doi: 10.1016/j.virusres.2008.07.007. Epub 2008 Aug 15. Virus Res. 2009. PMID: 18672012 Free PMC article. Review.
Cited by
-
The DNA virus white spot syndrome virus uses an internal ribosome entry site for translation of the highly expressed nonstructural protein ICP35.J Virol. 2013 Dec;87(24):13263-78. doi: 10.1128/JVI.01732-13. Epub 2013 Oct 2. J Virol. 2013. PMID: 24089551 Free PMC article.
-
Regulatory Roles of Rpl22 in Hematopoiesis: An Old Dog with New Tricks.Crit Rev Immunol. 2015;35(5):379-400. doi: 10.1615/critrevimmunol.v35.i5.30. Crit Rev Immunol. 2015. PMID: 26853850 Free PMC article. Review.
-
Conservation and diversity among the three-dimensional folds of the Dicistroviridae intergenic region IRESes.J Mol Biol. 2007 Jul 27;370(5):856-69. doi: 10.1016/j.jmb.2007.04.076. Epub 2007 May 8. J Mol Biol. 2007. PMID: 17544444 Free PMC article.
-
Modular domains of the Dicistroviridae intergenic internal ribosome entry site.RNA. 2010 Jun;16(6):1182-95. doi: 10.1261/rna.2044610. Epub 2010 Apr 27. RNA. 2010. PMID: 20423979 Free PMC article.
-
Translation from the Ribosome to the Clinic: Implication in Neurological Disorders and New Perspectives from Recent Advances.Biomolecules. 2019 Nov 1;9(11):680. doi: 10.3390/biom9110680. Biomolecules. 2019. PMID: 31683805 Free PMC article. Review.
References
-
- Christian P, Carstens E, Domier L, Johnson L, Johnson K, Nakashima N, Scotti P, van der Wilk F. Dicistroviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus Taxonomy VIII. San Diego: Elsevier Academic Press; 2005. pp. 783–788.
-
- Wilson JE, Pestova TV, Hellen CUT, Sarnow P. Initiation of protein synthesis from the A site of the ribosome. Cell. 2000;102:511–520. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

