Patients with ARDS show improvement but not normalisation of alveolar surface activity with surfactant treatment: putative role of neutral lipids
- PMID: 17287298
- PMCID: PMC2117258
- DOI: 10.1136/thx.2006.062398
Patients with ARDS show improvement but not normalisation of alveolar surface activity with surfactant treatment: putative role of neutral lipids
Abstract
Background: Extensive biochemical and biophysical changes of the pulmonary surfactant system occur in the acute respiratory distress syndrome (ARDS).
Methods: The effect of intrabronchial administration of a recombinant surfactant protein C-based surfactant preparation (Venticute) on gas exchange, surfactant composition and function was investigated in 31 patients with ARDS in a randomised controlled phase I/II clinical pilot trial. Bronchoalveolar lavage fluids for surfactant analysis were obtained 3 h before and 48 and 120 h after the first surfactant application. Potentially deleterious effects of surfactant neutral lipids in patients with ARDS were also identified.
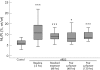
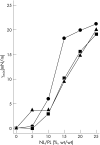
Results: Before treatment all patients had marked abnormalities in the surfactant phospholipid and protein composition. In response to surfactant treatment, gas exchange improved and surfactant phospholipid and protein content were almost normalised. Alveolar surface activity was dramatically impaired before treatment and only partially improved after surfactant administration. Further analysis of the bronchoalveolar lavage fluids revealed a twofold increase in neutral lipid content and altered neutral lipid profile in patients with ARDS compared with healthy controls. These differences persisted even after administration of large amounts of Venticute. Supplementation of Venticute or natural surfactant with a synthetic neutral lipid preparation, mimicking the profile in ARDS, caused a dose-dependent deterioration of surface activity in vitro.
Conclusion: Intrabronchial surfactant treatment improves gas exchange in ARDS, but the efficacy may be limited by increased concentration and altered neutral lipid profile in surfactant under these conditions.
Conflict of interest statement
Competing interests: WS receives grant and contract support and fees for consulting services by the following companies: Schering AG, Pfizer Ltd, Altana Pharma AG, Lung Rx, Myogen. None of the other authors has any financial relationship with a commercial entity that has an interest in the subject matter or materials discussed in the manuscript.
References
-
- Harwood J L. Lung surfactant. Prog Lipid Res 198726211–256. - PubMed
-
- Possmayer F. A proposed nomenclature for pulmonary surfactant‐associated proteins. Am Rev Respir Dis 1988138990–998. - PubMed
-
- Gross N J, Narine K R. Surfactant subtypes in mice: characterization and quantitation. J Appl Physiol 198966342–349. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources