Avidity of the immunoglobulin G response to a Neisseria meningitidis group C polysaccharide conjugate vaccine as measured by inhibition and chaotropic enzyme-linked immunosorbent assays
- PMID: 17287312
- PMCID: PMC1865604
- DOI: 10.1128/CVI.00241-06
Avidity of the immunoglobulin G response to a Neisseria meningitidis group C polysaccharide conjugate vaccine as measured by inhibition and chaotropic enzyme-linked immunosorbent assays
Abstract
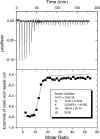
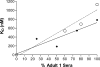
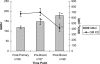
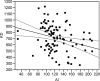
Antibody avidity, the strength of the multivalent interaction between antibodies and their antigens, is an important characteristic of protective immune responses. We have developed an inhibition enzyme-linked immunosorbent assay (ELISA) to measure antibody avidity for the capsular polysaccharide (PS) of Neisseria meningitidis group C (MnC) and determined the avidity constants (K(D)s) for 100 sera from children immunized with an MnC PS conjugate vaccine. The avidity constants were compared to the avidity indices (AI) obtained for the same sera using a chaotropic ELISA protocol. After the primary immunization series, the geometric mean (GM) K(D) was 674 nM and did not change in the months following immunization. However, the GM avidity did increase after the booster dose (GM K(D), 414 nM 1 month after booster immunization). In contrast, the GM AI increased from an initial value of 118 after the primary immunization series to 147 6 months after the completion of the primary immunization series and then further increased to 178 after booster immunization. At the individual subject level, the avidity constant and AI correlated after the primary immunization series and after booster immunization but not prior to boosting. This work suggests that the AI, as measured by the chaotropic ELISA, in contrast to the K(D), reflects changes that render antibody populations less susceptible to disruption by chaotropic agents without directly affecting the strength of the binding interactions.
Figures

Similar articles
-
Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with different neisseria meningitidis serogroup C conjugate vaccines.Pediatr Infect Dis J. 2009 Apr;28(4 Suppl):S77-88. doi: 10.1097/INF.0b013e318199f609. Pediatr Infect Dis J. 2009. PMID: 19325450 Clinical Trial.
-
Avidity of serogroup A meningococcal IgG antibodies after immunization with different doses of a tetravalent A/C/Y/W135 polysaccharide vaccine.Scand J Immunol. 2011 Jul;74(1):87-94. doi: 10.1111/j.1365-3083.2011.02535.x. Scand J Immunol. 2011. PMID: 21332570
-
Kinetics of antibody responses after primary immunization with meningococcal serogroup C conjugate vaccine or secondary immunization with either conjugate or polysaccharide vaccine in adults.Vaccine. 2009 Nov 23;27(50):6974-82. doi: 10.1016/j.vaccine.2009.09.082. Epub 2009 Oct 1. Vaccine. 2009. PMID: 19800445 Clinical Trial.
-
Immunogenicity of routinely used childhood vaccines when coadministered with the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV).Pediatr Infect Dis J. 2009 Apr;28(4 Suppl):S97-S108. doi: 10.1097/INF.0b013e318199f61b. Pediatr Infect Dis J. 2009. PMID: 19325452 Review.
-
Meningococcal disease: a review on available vaccines and vaccines in development.Minerva Med. 2007 Oct;98(5):575-89. Minerva Med. 2007. PMID: 18043565 Review.
Cited by
-
Immunogenicity of a peptide-based anti-IgE conjugate vaccine in non-human primates.Immun Inflamm Dis. 2016 Apr 1;4(2):135-147. doi: 10.1002/iid3.98. eCollection 2016 Jun. Immun Inflamm Dis. 2016. PMID: 27957325 Free PMC article.
-
Impaired antibody response to conjugated meningococcal serogroup C vaccine in asplenic patients.Eur J Clin Microbiol Infect Dis. 2011 May;30(5):611-8. doi: 10.1007/s10096-010-1129-2. Epub 2010 Dec 24. Eur J Clin Microbiol Infect Dis. 2011. PMID: 21184126
-
Identification of the smallest structure capable of evoking opsonophagocytic antibodies against Streptococcus pneumoniae type 14.Infect Immun. 2008 Oct;76(10):4615-23. doi: 10.1128/IAI.00472-08. Epub 2008 Aug 4. Infect Immun. 2008. PMID: 18678667 Free PMC article.
-
Thermodynamics and density of binding of a panel of antibodies to high-molecular-weight capsular polysaccharides.Clin Vaccine Immunol. 2009 Jan;16(1):37-42. doi: 10.1128/CVI.00290-08. Epub 2008 Nov 12. Clin Vaccine Immunol. 2009. PMID: 19005020 Free PMC article.
-
Timing of an adolescent booster after single primary meningococcal serogroup C conjugate immunization at young age; an intervention study among Dutch teenagers.PLoS One. 2014 Jun 25;9(6):e100651. doi: 10.1371/journal.pone.0100651. eCollection 2014. PLoS One. 2014. PMID: 24963638 Free PMC article.
References
-
- Anttila, M., J. Eskola, H. Ahman, and H. Kayhty. 1998. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J. Infect. Dis. 177:1614-1621. - PubMed
-
- Anttila, M., J. Eskola, H. Ahman, and H. Kayhty. 1999. Differences in the avidity of antibodies evoked by four different pneumococcal conjugate vaccines in early childhood. Vaccine 17:1970-1977. - PubMed
-
- Borrow, R., D. Goldblatt, N. Andrews, P. Richmond, J. Southern, and E. Miller. 2001. Influence of prior meningococcal C polysaccharide vaccination on the response and generation of memory after meningococcal C conjugate vaccination in young children. J. Infect. Dis. 184:377-380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

