Immunoblot assay using recombinant antigens as a supplemental test to confirm the presence of antibodies to Trypanosoma cruzi
- PMID: 17287316
- PMCID: PMC1865615
- DOI: 10.1128/CVI.00401-06
Immunoblot assay using recombinant antigens as a supplemental test to confirm the presence of antibodies to Trypanosoma cruzi
Abstract
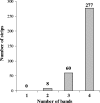
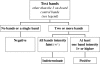
The diagnosis of chronic Chagas' disease is generally made by detecting antibodies to Trypanosoma cruzi. Most conventional serological tests are based on lysates of whole parasites or semipurified antigen fractions from T. cruzi epimastigotes grown in culture. The occurrence of inconclusive and false-positive results has been a persistent problem with the conventional assays, and there is no universally accepted gold standard for confirmation of positive test results. We describe here an immunoblot assay for detecting antibodies to T. cruzi in which four chimeric recombinant antigens (rAgs), designated FP3, FP6, FP10, and TcF, are used as target antigens. Each of these rAgs is composed of several antigenically distinct regions and includes repetitive as well as nonrepetitive sequences. Each rAg is coated as a discrete line on a nitrocellulose strip. Assay sensitivity was assessed by testing 345 specimens known to be positive for antibodies to T. cruzi. All 345 of these samples showed two to four reactive test bands in addition to the three on-board control bands that are on each strip. Assay specificity was determined by testing 500 specimens from random U.S. blood donors, all of which gave negative results. Based on the results obtained in this study, we propose the following scheme for interpretation of test results: (i) no bands or a single test band = a negative result; (ii) two or more test bands with at least one band showing intensity of 1+ or higher = a positive result; and (iii) multiple faint test bands (+/-) = indeterminate result. Based on this scheme, the prototype immunoblot assay showed sensitivity of 100% (n = 345) and specificity of 100% (n = 500). Additionally, all 269 potentially cross-reacting and T. cruzi antibody-negative specimens tested negative in our immunoblot assay. The rAg-based immunoblot assay has potential as a supplemental test for confirming the presence of antibodies to T. cruzi in blood specimens and for identifying false-positive results obtained with other assays.
Figures
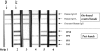
Similar articles
-
Enzyme-linked immunosorbent assay for serological diagnosis of Chagas' disease employing a Trypanosoma cruzi recombinant antigen that consists of four different peptides.J Clin Microbiol. 2001 Dec;39(12):4390-5. doi: 10.1128/JCM.39.12.4390-4395.2001. J Clin Microbiol. 2001. PMID: 11724850 Free PMC article.
-
Polymerase chain reaction reveals Trypanosoma cruzi infection suspected by serology in cutaneous and mucocutaneous leishmaniasis patients.Acta Trop. 1999 Apr 30;72(3):295-308. doi: 10.1016/s0001-706x(99)00005-4. Acta Trop. 1999. PMID: 10232785
-
Evaluation of a prototype Trypanosoma cruzi antibody assay with recombinant antigens on a fully automated chemiluminescence analyzer for blood donor screening.Transfusion. 2006 Oct;46(10):1737-44. doi: 10.1111/j.1537-2995.2006.00965.x. Transfusion. 2006. PMID: 17002630 Clinical Trial.
-
Serological diagnosis of Chagas disease with purified and defined Trypanosoma cruzi antigens.Mem Inst Oswaldo Cruz. 1999;94 Suppl 1:285-8. doi: 10.1590/s0074-02761999000700051. Mem Inst Oswaldo Cruz. 1999. PMID: 10677737 Review. No abstract available.
-
Diversity of Chagas disease diagnostic antigens: Successes and limitations.PLoS Negl Trop Dis. 2024 Oct 1;18(10):e0012512. doi: 10.1371/journal.pntd.0012512. eCollection 2024 Oct. PLoS Negl Trop Dis. 2024. PMID: 39352878 Free PMC article. Review.
Cited by
-
Towards a New Strategy for Diagnosis of Congenital Trypanosoma cruzi Infection.J Clin Microbiol. 2017 May;55(5):1396-1407. doi: 10.1128/JCM.02248-16. Epub 2017 Feb 15. J Clin Microbiol. 2017. PMID: 28202792 Free PMC article.
-
Diagnosis of parasitic diseases: old and new approaches.Interdiscip Perspect Infect Dis. 2009;2009:278246. doi: 10.1155/2009/278246. Epub 2009 Dec 30. Interdiscip Perspect Infect Dis. 2009. PMID: 20069111 Free PMC article.
-
A New Development in Trypanosoma cruzi Detection.J Clin Microbiol. 2017 Mar;55(3):690-692. doi: 10.1128/JCM.02353-16. Epub 2017 Jan 11. J Clin Microbiol. 2017. PMID: 28077696 Free PMC article.
-
Evaluation of the Chagas Western Blot IgG Assay for the Diagnosis of Chagas Disease.Pathogens. 2021 Nov 10;10(11):1455. doi: 10.3390/pathogens10111455. Pathogens. 2021. PMID: 34832611 Free PMC article.
-
Trypanosoma cruzi infection in transfusion medicine.Hematol Transfus Cell Ther. 2019 Jul-Sep;41(3):262-267. doi: 10.1016/j.htct.2018.12.001. Epub 2019 Mar 21. Hematol Transfus Cell Ther. 2019. PMID: 31085149 Free PMC article. Review.
References
-
- Almeida, I. C., D. T. Covas, L. M. T. Soussumi, and L. R. Travassos. 1997. A highly sensitive and specific chemiluminescent enzyme-linked immunosorbent assay for diagnosis of active Trypanosoma cruzi infection. Transfusion 37:850-857. - PubMed
-
- Anonymous. 2002. Control of Chagas disease. Technical report ser. 905. World Health Organization, Geneva, Switzerland. - PubMed
-
- Chang, C. D., K. Y. Cheng, L. Jiang, V. A. Salbilla, A. S. Haller, A. W. Yem, J. D. Bryant, L. V. Kirchhoff, D. A. Leiby, G. Schochetman, and D. O. Shah. 2006. Evaluation of a prototype Trypanosoma cruzi antibody assay with recombinant antigens on a fully automated chemiluminescence analyzer for blood donor screening. Transfusion 46:1737-1744. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

