Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion
- PMID: 17287343
- PMCID: PMC1794344
- DOI: 10.1073/pnas.0611134104
Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion
Abstract
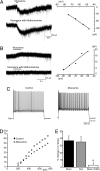
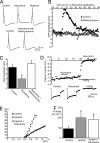
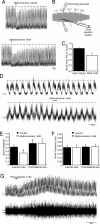
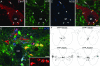
To effect movement, motoneurons must respond appropriately to motor commands. Their responsiveness to these inputs, or excitability, is regulated by neuromodulators. Possible sources of modulation include the abundant cholinergic "C boutons" that surround motoneuron somata. In the present study, recordings from motoneurons in spinal cord slices demonstrated that cholinergic activation of m2-type muscarinic receptors increases excitability by reducing the action potential afterhyperpolarization. Analyses of isolated spinal cord preparations in which fictive locomotion was elicited demonstrated that endogenous cholinergic inputs increase motoneuron excitability during locomotion. Anatomical data indicate that C boutons originate from a discrete group of interneurons lateral to the central canal, the medial partition neurons. These results highlight a unique component of spinal motor networks that is critical in ensuring that sufficient output is generated by motoneurons to drive motor behavior.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

