Deletion of CASK in mice is lethal and impairs synaptic function
- PMID: 17287346
- PMCID: PMC1892970
- DOI: 10.1073/pnas.0611003104
Deletion of CASK in mice is lethal and impairs synaptic function
Abstract
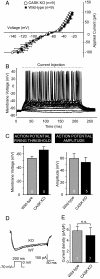
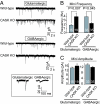
CASK is an evolutionarily conserved multidomain protein composed of an N-terminal Ca2+/calmodulin-kinase domain, central PDZ and SH3 domains, and a C-terminal guanylate kinase domain. Many potential activities for CASK have been suggested, including functions in scaffolding the synapse, in organizing ion channels, and in regulating neuronal gene transcription. To better define the physiological importance of CASK, we have now analyzed CASK "knockdown" mice in which CASK expression was suppressed by approximately 70%, and CASK knockout (KO) mice, in which CASK expression was abolished. CASK knockdown mice are viable but smaller than WT mice, whereas CASK KO mice die at first day after birth. CASK KO mice exhibit no major developmental abnormalities apart from a partially penetrant cleft palate syndrome. In CASK-deficient neurons, the levels of the CASK-interacting proteins Mints, Veli/Mals, and neurexins are decreased, whereas the level of neuroligin 1 (which binds to neurexins that in turn bind to CASK) is increased. Neurons lacking CASK display overall normal electrical properties and form ultrastructurally normal synapses. However, glutamatergic spontaneous synaptic release events are increased, and GABAergic synaptic release events are decreased in CASK-deficient neurons. In contrast to spontaneous neurotransmitter release, evoked release exhibited no major changes. Our data suggest that CASK, the only member of the membrane-associated guanylate kinase protein family that contains a Ca2+/calmodulin-dependent kinase domain, is required for mouse survival and performs a selectively essential function without being in itself required for core activities of neurons, such as membrane excitability, Ca2+-triggered presynaptic release, or postsynaptic receptor functions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Deficiency of calcium/calmodulin-dependent serine protein kinase disrupts the excitatory-inhibitory balance of synapses by down-regulating GluN2B.Mol Psychiatry. 2019 Jul;24(7):1079-1092. doi: 10.1038/s41380-018-0338-4. Epub 2019 Jan 4. Mol Psychiatry. 2019. PMID: 30610199 Free PMC article.
-
A role for Mints in transmitter release: Mint 1 knockout mice exhibit impaired GABAergic synaptic transmission.Proc Natl Acad Sci U S A. 2003 Feb 4;100(3):1409-14. doi: 10.1073/pnas.252774899. Epub 2003 Jan 23. Proc Natl Acad Sci U S A. 2003. PMID: 12547917 Free PMC article.
-
Membrane-tethered monomeric neurexin LNS-domain triggers synapse formation.J Neurosci. 2013 Sep 4;33(36):14617-28. doi: 10.1523/JNEUROSCI.1232-13.2013. J Neurosci. 2013. PMID: 24005312 Free PMC article.
-
The role of the MAGUK protein CASK in neural development and synaptic function.Curr Med Chem. 2006;13(16):1915-27. doi: 10.2174/092986706777585040. Curr Med Chem. 2006. PMID: 16842202 Review.
-
Structural constraints and functional divergences in CASK evolution.Biochem Soc Trans. 2013 Aug;41(4):1017-22. doi: 10.1042/BST20130061. Biochem Soc Trans. 2013. PMID: 23863172 Review.
Cited by
-
The Neurexin/N-Ethylmaleimide-sensitive Factor (NSF) Interaction Regulates Short Term Synaptic Depression.J Biol Chem. 2015 Jul 17;290(29):17656-17667. doi: 10.1074/jbc.M115.644583. Epub 2015 May 7. J Biol Chem. 2015. PMID: 25953899 Free PMC article.
-
An impaired splicing program underlies differentiation defects in hSOD1G93A neural progenitor cells.Cell Mol Life Sci. 2023 Jul 31;80(8):236. doi: 10.1007/s00018-023-04893-7. Cell Mol Life Sci. 2023. PMID: 37524863 Free PMC article.
-
Srsf3 mediates alternative RNA splicing downstream of PDGFRα signaling in the facial mesenchyme.Development. 2021 Jul 15;148(14):dev199448. doi: 10.1242/dev.199448. Epub 2021 Jul 13. Development. 2021. PMID: 34184034 Free PMC article.
-
The Non-Linear Path from Gene Dysfunction to Genetic Disease: Lessons from the MICPCH Mouse Model.Cells. 2022 Mar 28;11(7):1131. doi: 10.3390/cells11071131. Cells. 2022. PMID: 35406695 Free PMC article. Review.
-
Neuroligins and neurexins link synaptic function to cognitive disease.Nature. 2008 Oct 16;455(7215):903-11. doi: 10.1038/nature07456. Nature. 2008. PMID: 18923512 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

