The PsbQ protein defines cyanobacterial Photosystem II complexes with highest activity and stability
- PMID: 17287351
- PMCID: PMC1892988
- DOI: 10.1073/pnas.0609337104
The PsbQ protein defines cyanobacterial Photosystem II complexes with highest activity and stability
Abstract
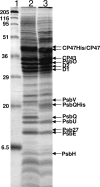
Light-induced conversion of water to molecular oxygen by Photosystem II (PSII) is one of the most important enzymatic reactions in the biosphere. PSII is a multisubunit membrane protein complex with numerous associated cofactors, but it continually undergoes assembly and disassembly due to frequent light-mediated damage as a result of its normal function. Thus, at any instant, there is heterogeneity in the subunit compositions of PSII complexes within the cell. In particular, cyanobacterial PSII complexes have five associated extrinsic proteins, PsbO, PsbP, PsbQ, PsbU, and PsbV. However, little is known about the interactions of the more recently identified PsbQ protein with other components in cyanobacterial PSII. Here we show that PSII complexes can be isolated from the cyanobacterium Synechocystis sp. PCC 6803 on the basis of the presence of a polyhistidine-tagged PsbQ protein. Purification of PSII complexes using a tagged extrinsic protein has not been previously described, and this work conclusively demonstrates that PsbQ is present in combination with the PsbO, PsbU, and PsbV proteins in cyanobacterial PSII. Moreover, PsbQ-associated PSII complexes have higher activity and stability relative to those isolated using histidine-tagged CP47, an integral membrane protein. Therefore, we conclude that the presence of PsbQ defines the fully assembled and optimally active form of the enzyme.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S. Science. 2004;303:1831–1838. - PubMed
-
- Loll B, Kern J, Saenger W, Zouni A, Biesiadka J. Nature. 2005;438:1040–1044. - PubMed
-
- Zouni A, Witt HT, Kern J, Fromme P, Krauss N, Saenger W, Orth P. Nature. 2001;409:739–743. - PubMed
-
- Aro EM, Suorsa M, Rokka A, Allahverdiyeva Y, Paakkarinen V, Saleem A, Battchikova N, Rintamaki E. J Exp Bot. 2005;56:347–356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

