Motor strategies used by rats spinalized at birth to maintain stance in response to imposed perturbations
- PMID: 17287444
- PMCID: PMC2919298
- DOI: 10.1152/jn.00308.2006
Motor strategies used by rats spinalized at birth to maintain stance in response to imposed perturbations
Abstract
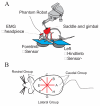
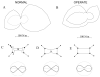
Some rats spinalized P1/P2 achieve autonomous weight-supported locomotion and quiet stance as adults. We used force platforms and robot-applied perturbations to test such spinalized rats (n = 6) that exhibited both weight-supporting locomotion and stance, and also normal rats (n = 8). Ground reaction forces in individual limbs and the animals' center of pressure were examined. In normal rats, both forelimbs and hindlimbs participated actively to control horizontal components of ground reaction forces. Rostral perturbations increased forelimb ground reaction forces and caudal perturbations increased hindlimb ground reaction forces. Operate rats carried 60% body weight on the forelimbs and had a more rostral center of pressure placement. The pattern in normal rats was to carry significantly more weight on the hindlimbs in quiet stance (roughly 60%). The strategy of operate rats to compensate for perturbations was entirely in forelimbs; as a result, the hindlimbs were largely isolated from the perturbation. Stiffness magnitude of the whole body was measured: its magnitude was hourglass shaped, with the principal axis oriented rostrocaudally. Operate rats were significantly less stiff--only 60-75% of normal rats' stiffness. The injured rats adopt a stance strategy that isolates the hindlimbs from perturbation and may thus prevent hindlimb loadings. Such loadings could initiate reflex stepping, which we observed. This might activate lumbar pattern generators used in their locomotion. Adult spinalized rats never achieve independent hindlimb weight-supported stance. The stance strategy of the P1 spinalized rats differed strongly from the behavior of intact rats and may be difficult for rats spinalized as adults to master.
Figures







Similar articles
-
Weight support and balance during perturbed stance in the chronic spinal cat.J Neurophysiol. 1999 Dec;82(6):3066-81. doi: 10.1152/jn.1999.82.6.3066. J Neurophysiol. 1999. PMID: 10601442
-
Attributes of quiet stance in the chronic spinal cat.J Neurophysiol. 1999 Dec;82(6):3056-65. doi: 10.1152/jn.1999.82.6.3056. J Neurophysiol. 1999. PMID: 10601441
-
Coordination strategies for limb forces during weight-bearing locomotion in normal rats, and in rats spinalized as neonates.Exp Brain Res. 2008 Sep;190(1):53-69. doi: 10.1007/s00221-008-1451-4. Epub 2008 Jul 9. Exp Brain Res. 2008. PMID: 18612631 Free PMC article.
-
Trunk sensorimotor cortex is essential for autonomous weight-supported locomotion in adult rats spinalized as P1/P2 neonates.J Neurophysiol. 2008 Aug;100(2):839-51. doi: 10.1152/jn.00866.2007. Epub 2008 May 28. J Neurophysiol. 2008. PMID: 18509082 Free PMC article.
-
[Neuronal control of posture and locomotion in decerebrated and spinalized animals].Ross Fiziol Zh Im I M Sechenova. 2013 Mar;99(3):392-405. Ross Fiziol Zh Im I M Sechenova. 2013. PMID: 23789442 Russian.
Cited by
-
A bipedal mammalian model for spinal cord injury research: The tammar wallaby.F1000Res. 2017 Jun 15;6:921. doi: 10.12688/f1000research.11712.1. eCollection 2017. F1000Res. 2017. PMID: 28721206 Free PMC article.
-
Postural control in paw distance after labyrinthectomy-induced vestibular imbalance.Med Biol Eng Comput. 2020 Dec;58(12):3039-3047. doi: 10.1007/s11517-020-02276-9. Epub 2020 Oct 20. Med Biol Eng Comput. 2020. PMID: 33079344
-
Exercise induces cortical plasticity after neonatal spinal cord injury in the rat.J Neurosci. 2009 Jun 10;29(23):7549-57. doi: 10.1523/JNEUROSCI.2474-08.2009. J Neurosci. 2009. PMID: 19515923 Free PMC article.
-
Spinal cord modularity: evolution, development, and optimization and the possible relevance to low back pain in man.Exp Brain Res. 2010 Jan;200(3-4):283-306. doi: 10.1007/s00221-009-2016-x. Epub 2009 Oct 9. Exp Brain Res. 2010. PMID: 19838690 Free PMC article. Review. No abstract available.
-
The upright posture improves plantar stepping and alters responses to serotonergic drugs in spinal rats.J Physiol. 2012 Apr 1;590(7):1721-36. doi: 10.1113/jphysiol.2011.224931. Epub 2012 Feb 20. J Physiol. 2012. PMID: 22351637 Free PMC article.
References
-
- Collins JJ, De Luca CJ. Upright, correlated random walks: A statistical-biomechanics approach to the human postural control system. Chaos. 1995 Mar;5(1):57–63. - PubMed
-
- Collins JJ, De Luca CJ. Open-loop and closed-loop control of posture: a random-walk analysis of center-of-pressure trajectories. Exp Brain Res. 1993;95(2):308–18. - PubMed
-
- de Leon RD, Kubasak MD, Phelps PE, Timoszyk WK, Reinkensmeyer DJ, Roy RR, Edgerton VR. Using robotics to teach the spinal cord to walk. Brain Res Brain Res Rev. 2002a;40(1-3):267–73. - PubMed
-
- de Leon RD, Reinkensmeyer DJ, Timoszyk WK, London NJ, Roy RR, Edgerton VR. Use of robotics in assessing the adaptive capacity of the rat lumbar spinal cord. Prog Brain Res. 2002b;137:141–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

