Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients
- PMID: 17287462
- PMCID: PMC2995532
- DOI: 10.2337/db06-0783
Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients
Abstract
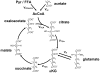
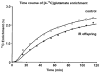
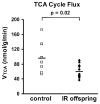
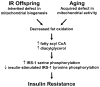
Insulin resistance is the best predictor for the development of diabetes in offspring of type 2 diabetic patients, but the mechanism responsible for it remains unknown. Recent studies have demonstrated increased intramyocellular lipid, decreased mitochondrial ATP synthesis, and decreased mitochondrial density in the muscle of lean, insulin-resistant offspring of type 2 diabetic patients. These data suggest an important role for mitochondrial dysfunction in the pathogenesis of type 2 diabetes. To further explore this hypothesis, we assessed rates of substrate oxidation in the muscle of these same individuals using (13)C magnetic resonance spectroscopy (MRS). Young, lean, insulin-resistant offspring of type 2 diabetic patients and insulin-sensitive control subjects underwent (13)C MRS studies to noninvasively assess rates of substrate oxidation in muscle by monitoring the incorporation of (13)C label into C(4) glutamate during a [2-(13)C]acetate infusion. Using this approach, we found that rates of muscle mitochondrial substrate oxidation were decreased by 30% in lean, insulin-resistant offspring (59.8 +/- 5.1 nmol x g(-1) x min(-1), P = 0.02) compared with insulin-sensitive control subjects (96.1 +/- 16.3 nmol x g(-1) x min(-1)). These data support the hypothesis that insulin resistance in skeletal muscle of insulin-resistant offspring is associated with dysregulation of intramyocellular fatty acid metabolism, possibly because of an inherited defect in the activity of mitochondrial oxidative phosphorylation.
Figures
References
-
- Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR. Slow glucose removal and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med. 1990;113:909–915. - PubMed
-
- Phillips DI, Caddy S, Ilic V, Fielding BA, Frayn KN, Borthwick AC, Taylor R. Intramuscular triglyceride and muscle insulin sensitivity: evidence for a relationship in non-diabetic subjects. Metabolism. 1996;45:947–950. - PubMed
-
- Krssak M, Petersen KF, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. - PubMed
-
- Szczepaniak LS, Babcock EE, Schick F, Dobbins RL, Garg A, Burns DK, McGarry JD, Stein DT. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol Endocrinol Metab. 1999;276:E977–E989. - PubMed
-
- Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcellono C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48:1600–1606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

