The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation
- PMID: 17287498
- PMCID: PMC6673586
- DOI: 10.1523/JNEUROSCI.5320-06.2007
The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation
Abstract
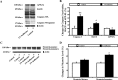
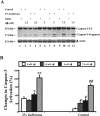
The anesthetic isoflurane has been reported to induce apoptosis and increase Abeta generation and aggregation. However, the molecular mechanism underlying these effects remains unknown. We therefore set out to assess whether the effects of isoflurane on apoptosis are linked to amyloid beta-protein (Abeta) generation and aggregation. For this purpose, we assessed the effects of isoflurane on beta-site amyloid beta precursor protein (APP)-cleaving enzyme (BACE) and gamma-secretase, the proteases responsible for Abeta generation. We also tested the effects of inhibitors of Abeta aggregation (iAbeta5, a beta-sheet breaker peptide; clioquinol, a copper-zinc chelator) on the ability of isoflurane to induce apoptosis. All of these studies were performed on naive human H4 neuroglioma cells as well as those overexpressing APP (H4-APP cells). Isoflurane increased the levels of BACE and gamma-secretase and secreted Abeta in the H4-APP cells. Isoflurane-induced Abeta generation could be blocked by the broad-based caspase inhibitor Z-VAD. The Abeta aggregation inhibitors, iAbeta5 and clioquinol, selectively attenuated caspase-3 activation induced by isoflurane. However, isoflurane was able to induce caspase-3 activation in the absence of any detectable alterations of Abeta generation in naive H4 cells. Finally, Abeta potentiated the isoflurane-induced caspase-3 activation in naive H4 cells. Collectively, these findings suggest that isoflurane can induce apoptosis, which, in turn, increases BACE and gamma-secretase levels and Abeta secretion. Isoflurane also promotes Abeta aggregation. Accumulation of aggregated Abeta in the media can then promote apoptosis. The result is a vicious cycle of isoflurane-induced apoptosis, Abeta generation and aggregation, and additional rounds of apoptosis, leading to cell death.
Figures




References
-
- Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron. 2001;30:665–676. - PubMed
-
- De Felice FG, Vieira MN, Saraiva LM, Figueroa-Villar JD, Garcia-Abreu J, Liu R, Chang L, Klein WL, Ferreira ST. Targeting the neurotoxic species in Alzheimer's disease: inhibitors of Abeta oligomerization. FASEB J. 2004;18:1366–1372. - PubMed
-
- Eckenhoff RG, Johansson JS, Wei H, Carnini A, Kang B, Wei W, Pidikiti R, Keller JM, Eckenhoff MF. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–709. - PubMed
-
- Florent S, Malaplate-Armand C, Youssef I, Kriem B, Koziel V, Escanye MC, Fifre A, Sponne I, Leininger-Muller B, Olivier JL, Pillot T, Oster T. Docosahexaenoic acid prevents neuronal apoptosis induced by soluble amyloid-beta oligomers. J Neurochem. 2006;96:385–395. - PubMed
-
- Francis R, McGrath G, Zhang J, Ruddy DA, Sym M, Apfeld J, Nicoll M, Maxwell M, Hai B, Ellis MC, Parks AL, Xu W, Li J, Gurney M, Myers RL, Himes CS, Hiebsch R, Ruble C, Nye JS, Curtis D. aph-1 and pen-2 are required for Notch pathway signaling, gamma-secretase cleavage of betaAPP, and presenilin protein accumulation. Dev Cell. 2002;3:85–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
