Spike timing-dependent synaptic depression in the in vivo barrel cortex of the rat
- PMID: 17287502
- PMCID: PMC3070399
- DOI: 10.1523/JNEUROSCI.4264-06.2007
Spike timing-dependent synaptic depression in the in vivo barrel cortex of the rat
Abstract
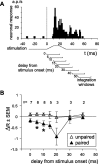
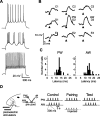
Spike timing-dependent plasticity (STDP) is a computationally powerful form of plasticity in which synapses are strengthened or weakened according to the temporal order and precise millisecond-scale delay between presynaptic and postsynaptic spiking activity. STDP is readily observed in vitro, but evidence for STDP in vivo is scarce. Here, we studied spike timing-dependent synaptic depression in single putative pyramidal neurons of the rat primary somatosensory cortex (S1) in vivo, using two techniques. First, we recorded extracellularly from layer 2/3 (L2/3) and L5 neurons, and paired spontaneous action potentials (postsynaptic spikes) with subsequent subthreshold deflection of one whisker (to drive presynaptic afferents to the recorded neuron) to produce "post-leading-pre" spike pairings at known delays. Short delay pairings (<17 ms) resulted in a significant decrease of the extracellular spiking response specific to the paired whisker, consistent with spike timing-dependent synaptic depression. Second, in whole-cell recordings from neurons in L2/3, we paired postsynaptic spikes elicited by direct-current injection with subthreshold whisker deflection to drive presynaptic afferents to the recorded neuron at precise temporal delays. Post-leading-pre pairing (<33 ms delay) decreased the slope and amplitude of the PSP evoked by the paired whisker, whereas "pre-leading-post" delays failed to produce depression, and sometimes produced potentiation of whisker-evoked PSPs. These results demonstrate that spike timing-dependent synaptic depression occurs in S1 in vivo, and is therefore a plausible plasticity mechanism in the sensory cortex.
Figures







Similar articles
-
Developmental switch in spike timing-dependent plasticity at layers 4-2/3 in the rodent barrel cortex.J Neurosci. 2012 Oct 24;32(43):15000-11. doi: 10.1523/JNEUROSCI.2506-12.2012. J Neurosci. 2012. PMID: 23100422 Free PMC article.
-
Dynamic representation of whisker deflection by synaptic potentials in spiny stellate and pyramidal cells in the barrels and septa of layer 4 rat somatosensory cortex.J Physiol. 2002 Aug 15;543(Pt 1):49-70. doi: 10.1113/jphysiol.2002.018465. J Physiol. 2002. PMID: 12181281 Free PMC article.
-
Spatiotemporal dynamics of sensory responses in layer 2/3 of rat barrel cortex measured in vivo by voltage-sensitive dye imaging combined with whole-cell voltage recordings and neuron reconstructions.J Neurosci. 2003 Feb 15;23(4):1298-309. doi: 10.1523/JNEUROSCI.23-04-01298.2003. J Neurosci. 2003. PMID: 12598618 Free PMC article.
-
A Hypothetical Model Concerning How Spike-Timing-Dependent Plasticity Contributes to Neural Circuit Formation and Initiation of the Critical Period in Barrel Cortex.J Neurosci. 2019 May 15;39(20):3784-3791. doi: 10.1523/JNEUROSCI.1684-18.2019. Epub 2019 Mar 15. J Neurosci. 2019. PMID: 30877173 Free PMC article. Review.
-
Population coding in somatosensory cortex.Curr Opin Neurobiol. 2002 Aug;12(4):441-7. doi: 10.1016/s0959-4388(02)00338-0. Curr Opin Neurobiol. 2002. PMID: 12139993 Review.
Cited by
-
Electrophysiological characterization of spino-sciatic and cortico-sciatic associative plasticity: modulation by trans-spinal direct current and effects on recovery after spinal cord injury in mice.J Neurosci. 2013 Mar 13;33(11):4935-46. doi: 10.1523/JNEUROSCI.4930-12.2013. J Neurosci. 2013. PMID: 23486964 Free PMC article.
-
Information Carried by Population Spike Times in the Whisker Sensory Cortex can be Decoded Without Knowledge of Stimulus Time.Front Synaptic Neurosci. 2010 Jun 14;2:17. doi: 10.3389/fnsyn.2010.00017. eCollection 2010. Front Synaptic Neurosci. 2010. PMID: 21423503 Free PMC article.
-
Learning by stimulation avoidance: A principle to control spiking neural networks dynamics.PLoS One. 2017 Feb 3;12(2):e0170388. doi: 10.1371/journal.pone.0170388. eCollection 2017. PLoS One. 2017. PMID: 28158309 Free PMC article.
-
Adult plasticity in multisensory neurons: short-term experience-dependent changes in the superior colliculus.J Neurosci. 2009 Dec 16;29(50):15910-22. doi: 10.1523/JNEUROSCI.4041-09.2009. J Neurosci. 2009. PMID: 20016107 Free PMC article.
-
Microcircuit mechanisms involved in paired associative stimulation-induced depression of corticospinal excitability.J Physiol. 2013 Oct 1;591(19):4903-20. doi: 10.1113/jphysiol.2013.253989. Epub 2013 Jul 15. J Physiol. 2013. PMID: 23858008 Free PMC article.
References
-
- Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;(Suppl):1178–1183. - PubMed
-
- Armstrong-James M, Fox K, Das-Gupta A. Flow of excitation within rat barrel cortex on striking a single vibrissa. J Neurophysiol. 1992;68:1345–1358. - PubMed
-
- Bear MF, Abraham WC. Long-term depression in hippocampus. Annu Rev Neurosci. 1996;19:437–462. - PubMed
-
- Bell CC, Han VZ, Sugawara Y, Grant K. Synaptic plasticity in a cerebellum-like structure depends on temporal order. Nature. 1997;387:278–281. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
