A reevaluation of the inverse dynamic model for eye movements
- PMID: 17287509
- PMCID: PMC6673592
- DOI: 10.1523/JNEUROSCI.3822-06.2007
A reevaluation of the inverse dynamic model for eye movements
Abstract
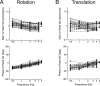
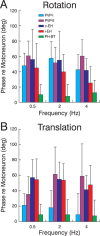
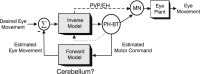
To construct an appropriate motor command from signals that provide a representation of desired action, the nervous system must take into account the dynamic characteristics of the motor plant to be controlled. In the oculomotor system, signals specifying desired eye velocity are thought to be transformed into motor commands by an inverse dynamic model of the eye plant that is shared for all types of eye movements and implemented by a weighted combination of eye velocity and position signals. Neurons in the prepositus hypoglossi and adjacent medial vestibular nuclei (PH-BT neurons) were traditionally thought to encode the "eye position" component of this inverse model. However, not only are PH-BT responses inconsistent with this theoretical role, but compensatory eye movement responses to translation do not show evidence for processing by a common inverse dynamic model. Prompted by these discrepancies between theoretical notions and experimental observations, we reevaluated these concepts using multiple-frequency rotational and translational head movements. Compatible with the notion of a common inverse model, we show that PH-BT responses are unique among all premotor cell types in bearing a consistent relationship to the motor output during eye movements driven by different sensory stimuli. However, because their responses are dynamically identical to those of motoneurons, PH-BT neurons do not simply represent an internal component of the inverse model, but rather its output. They encode and distribute an estimate of the motor command, a signal critical for accurate motor execution and learning.
Figures






Similar articles
-
Discharge patterns in nucleus prepositus hypoglossi and adjacent medial vestibular nucleus during horizontal eye movement in behaving macaques.J Neurophysiol. 1992 Jul;68(1):319-32. doi: 10.1152/jn.1992.68.1.319. J Neurophysiol. 1992. PMID: 1517825
-
Differential sensorimotor processing of vestibulo-ocular signals during rotation and translation.J Neurosci. 2001 Jun 1;21(11):3968-85. doi: 10.1523/JNEUROSCI.21-11-03968.2001. J Neurosci. 2001. PMID: 11356885 Free PMC article.
-
Neural correlates of forward and inverse models for eye movements: evidence from three-dimensional kinematics.J Neurosci. 2008 May 7;28(19):5082-7. doi: 10.1523/JNEUROSCI.0513-08.2008. J Neurosci. 2008. PMID: 18463261 Free PMC article.
-
[Central oculomotor circuits].Rev Neurol (Paris). 1985;141(5):349-70. Rev Neurol (Paris). 1985. PMID: 3901182 Review. French.
-
Basic organization principles of the VOR: lessons from frogs.Prog Neurobiol. 2004 Jul;73(4):259-309. doi: 10.1016/j.pneurobio.2004.05.003. Prog Neurobiol. 2004. PMID: 15261395 Review.
Cited by
-
Internal models of eye movement in the floccular complex of the monkey cerebellum.Neuroscience. 2009 Sep 1;162(3):763-76. doi: 10.1016/j.neuroscience.2009.03.059. Epub 2009 Mar 29. Neuroscience. 2009. PMID: 19336251 Free PMC article. Review.
-
Neural representation of orientation relative to gravity in the macaque cerebellum.Neuron. 2013 Dec 18;80(6):1508-18. doi: 10.1016/j.neuron.2013.09.029. Neuron. 2013. PMID: 24360549 Free PMC article.
-
Does orbital proprioception contribute to gaze stability during translation?Exp Brain Res. 2011 Nov;215(1):77-87. doi: 10.1007/s00221-011-2873-y. Epub 2011 Sep 27. Exp Brain Res. 2011. PMID: 21947173
-
A Neuroanatomically Grounded Optimal Control Model of the Compensatory Eye Movement System in Mice.Front Syst Neurosci. 2020 Mar 25;14:13. doi: 10.3389/fnsys.2020.00013. eCollection 2020. Front Syst Neurosci. 2020. PMID: 32269516 Free PMC article.
-
Contributions of ocular vestibular evoked myogenic potentials and the electrooculogram to periocular potentials produced by whole-body vibration.J Appl Physiol (1985). 2012 Nov;113(10):1613-23. doi: 10.1152/japplphysiol.00375.2012. Epub 2012 Sep 13. J Appl Physiol (1985). 2012. PMID: 22984251 Free PMC article.
References
-
- Angelaki DE. Three-dimensional organization of otolith-ocular reflexes in rhesus monkeys. III. Responses to translation. J Neurophysiol. 1998;80:680–695. - PubMed
-
- Angelaki DE, McHenry MQ, Hess BJ. Primate translational vestibulo-ocular reflexes. I. High-frequency dynamics and three-dimensional properties during lateral motion. J Neurophysiol. 2000;83:1637–1647. - PubMed
-
- Arnold DB, Robinson DA. A learning network model of the neural integrator of the oculomotor system. Biol Cybern. 1991;64:447–454. - PubMed
-
- Arnold DB, Robinson DA. The oculomotor integrator: testing of a neural network model. Exp Brain Res. 1997;113:57–74. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
