Erbin enhances voltage-dependent facilitation of Ca(v)1.3 Ca2+ channels through relief of an autoinhibitory domain in the Ca(v)1.3 alpha1 subunit
- PMID: 17287512
- PMCID: PMC6673595
- DOI: 10.1523/JNEUROSCI.5191-06.2007
Erbin enhances voltage-dependent facilitation of Ca(v)1.3 Ca2+ channels through relief of an autoinhibitory domain in the Ca(v)1.3 alpha1 subunit
Abstract
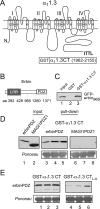
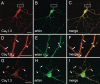
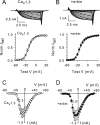
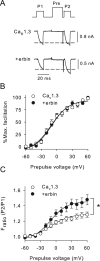
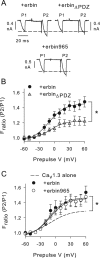
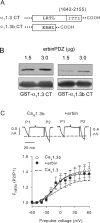
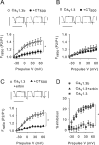
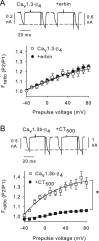
Ca(v)1.3 (L-type) voltage-gated Ca2+ channels have emerged as key players controlling Ca2+ signals at excitatory synapses. Compared with the more widely expressed Ca(v)1.2 L-type channel, relatively little is known about the mechanisms that regulate Ca(v)1.3 channels. Here, we describe a new role for the PSD-95 (postsynaptic density-95)/Discs large/ZO-1 (zona occludens-1) (PDZ) domain-containing protein, erbin, in directly potentiating Ca(v)1.3. Erbin specifically forms a complex with Ca(v)1.3, but not Ca(v)1.2, in transfected cells. The significance of erbin/Ca(v)1.3 interactions is supported by colocalization in somatodendritic domains of cortical neurons in culture and coimmunoprecipitation from rat brain lysates. In electrophysiological recordings, erbin augments facilitation of Ca(v)1.3 currents by a conditioning prepulse, a process known as voltage-dependent facilitation (VDF). This effect requires a direct interaction of the erbin PDZ domain with a PDZ recognition site in the C-terminal domain (CT) of the long variant of the Ca(v)1.3 alpha1 subunit (alpha1 1.3). Compared with Ca(v)1.3, the Ca(v)1.3b splice variant, which lacks a large fraction of the alpha1 1.3 CT, shows robust VDF that is not further affected by erbin. When coexpressed as an independent entity with Ca(v)1.3b or Ca(v)1.3 plus erbin, the alpha1 1.3 CT strongly suppresses VDF, signifying an autoinhibitory function of this part of the channel. These modulatory effects of erbin, but not alpha1 1.3 CT, depend on the identity of the auxiliary Ca2+ channel beta subunit. Our findings reveal a novel mechanism by which PDZ interactions and alternative splicing of alpha1 1.3 may influence activity-dependent regulation of Ca(v)1.3 channels at the synapse.
Figures


Similar articles
-
G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain.J Neurosci. 2005 Feb 2;25(5):1050-62. doi: 10.1523/JNEUROSCI.3327-04.2005. J Neurosci. 2005. PMID: 15689540 Free PMC article.
-
Functional diversity in neuronal voltage-gated calcium channels by alternative splicing of Ca(v)alpha1.Mol Neurobiol. 2002 Aug;26(1):21-44. doi: 10.1385/MN:26:1:021. Mol Neurobiol. 2002. PMID: 12392054 Review.
-
Association of CaV1.3 L-type calcium channels with Shank.J Neurosci. 2005 Feb 2;25(5):1037-49. doi: 10.1523/JNEUROSCI.4554-04.2005. J Neurosci. 2005. PMID: 15689539 Free PMC article.
-
Ca2+-binding protein-1 facilitates and forms a postsynaptic complex with Cav1.2 (L-type) Ca2+ channels.J Neurosci. 2004 May 12;24(19):4698-708. doi: 10.1523/JNEUROSCI.5523-03.2004. J Neurosci. 2004. PMID: 15140941 Free PMC article.
-
Structures and functions of calcium channel beta subunits.J Bioenerg Biomembr. 1998 Aug;30(4):357-75. doi: 10.1023/a:1021989622656. J Bioenerg Biomembr. 1998. PMID: 9758332 Review.
Cited by
-
Harmonin enhances voltage-dependent facilitation of Cav1.3 channels and synchronous exocytosis in mouse inner hair cells.J Physiol. 2013 Jul 1;591(13):3253-69. doi: 10.1113/jphysiol.2013.254367. Epub 2013 Apr 22. J Physiol. 2013. PMID: 23613530 Free PMC article.
-
Development of phenotypic assays for identifying novel blockers of L-type calcium channels in neurons.Sci Rep. 2021 Jan 11;11(1):456. doi: 10.1038/s41598-020-80692-5. Sci Rep. 2021. PMID: 33432098 Free PMC article.
-
Nrg1/ErbB signaling networks in Schwann cell development and myelination.Semin Cell Dev Biol. 2010 Dec;21(9):922-8. doi: 10.1016/j.semcdb.2010.08.008. Epub 2010 Sep 9. Semin Cell Dev Biol. 2010. PMID: 20832498 Free PMC article. Review.
-
Channelopathies in Cav1.1, Cav1.3, and Cav1.4 voltage-gated L-type Ca2+ channels.Pflugers Arch. 2010 Jul;460(2):361-74. doi: 10.1007/s00424-010-0800-x. Epub 2010 Mar 7. Pflugers Arch. 2010. PMID: 20213496 Free PMC article. Review.
-
The leucine-rich repeat signaling scaffolds Shoc2 and Erbin: cellular mechanism and role in disease.FEBS J. 2021 Feb;288(3):721-739. doi: 10.1111/febs.15450. Epub 2020 Jul 6. FEBS J. 2021. PMID: 32558243 Free PMC article. Review.
References
-
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–186. - PubMed
-
- Borg JP, Marchetto S, Le Bivic A, Ollendorff V, Jaulin-Bastard F, Saito H, Fournier E, Adelaide J, Margolis B, Birnbaum D. ERBIN: a basolateral PDZ protein that interacts with the mammalian ERBB2/HER2 receptor. Nat Cell Biol. 2000;2:407–414. - PubMed
-
- Burgess DL, Jones JM, Meisler MH, Noebels JL. Mutation of the Ca2+ channel β subunit gene Cchb4 is associated with ataxia and seizures in the lethargic (lh) mouse. Cell. 1997;88:385–392. - PubMed
-
- Burgess DL, Biddlecome GH, McDonough SI, Diaz ME, Zilinski CA, Bean BP, Campbell KP, Noebels JL. β subunit reshuffling modifies N- and P/Q-type Ca2+ channel subunit compositions in lethargic mouse brain. Mol Cell Neurosci. 1999;13:293–311. - PubMed
-
- Castellano A, Wei X, Birnbaumer L, Perez-Reyes E. Cloning and expression of a neuronal calcium channel β subunit. J Biol Chem. 1993;268:12359–12366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
