Primed vesicles can be distinguished from docked vesicles by analyzing their mobility
- PMID: 17287513
- PMCID: PMC6673599
- DOI: 10.1523/JNEUROSCI.4714-06.2007
Primed vesicles can be distinguished from docked vesicles by analyzing their mobility
Abstract
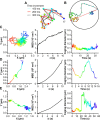
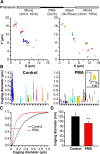
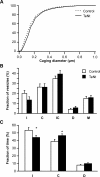
Neurotransmitters are released from nerve terminals and neuroendocrine cells by calcium-dependent exocytosis of vesicles. Before fusion, vesicles are docked to the plasma membrane and rendered release competent through a process called priming. Electrophysiological methods such as membrane capacitance measurements and carbon fiber amperometry accurately measure the fusion step of exocytosis with high time resolution but provide only indirect information about priming and docking. Total internal reflection fluorescence microscopy (TIRFM) enables the real-time visualization of vesicles, near the plasma membrane, as they undergo changes from one molecular state to the other. We devised a new method to analyze the mobility of vesicles, which not only allowed us to classify the movement of vesicles in three different categories but also to monitor dynamic changes in the mobility of vesicles over time. We selectively enhanced priming by treating bovine chromaffin cells with phorbol myristate acetate (PMA) or by overexpressing Munc13-1 (mammalian Unc) and analyzed the mobility of large dense-core vesicles. We demonstrate that nearly immobile vesicles represent primed vesicles because the pool of vesicles displaying this type of mobility was significantly increased after PMA treatment and Munc13-1 overexpression and decreased during tetanus toxin expression. Moreover, we showed that the movement of docked but unprimed vesicles is restricted to a confined region of approximately 220 nm diameter. Finally, a small third population of undocked vesicles showed a directed and probably active type of mobility. For the first time, we can thus distinguish the molecular state of vesicles in TIRFM by their mobility.
Figures




References
-
- Ashery U, Betz A, Xu T, Brose N, Rettig J. An efficient method for infection of adrenal chromaffin cells using the Semliki Forest virus gene expression system. Eur J Cell Biol. 1999;78:525–532. - PubMed
-
- Becherer U, Rettig J. Vesicle pools, docking, priming and release. Cell Tiss Res. 2006;326:393–407. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
